[English] 日本語
 Yorodumi
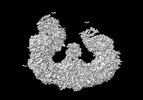
Yorodumi- PDB-8atm: Structure of the giant inhibitor of apoptosis, BIRC6 (composite map) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8atm | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of the giant inhibitor of apoptosis, BIRC6 (composite map) | ||||||
 Components Components | Baculoviral IAP repeat-containing protein 6 | ||||||
 Keywords Keywords |  APOPTOSIS / E2/E3 ubiquitin ligase APOPTOSIS / E2/E3 ubiquitin ligase | ||||||
| Function / homology |  Function and homology information Function and homology informationspongiotrophoblast layer development / labyrinthine layer development / ALK mutants bind TKIs / Flemming body /  microtubule organizing center / cysteine-type endopeptidase inhibitor activity / ubiquitin conjugating enzyme activity / Signaling by ALK fusions and activated point mutants / microtubule organizing center / cysteine-type endopeptidase inhibitor activity / ubiquitin conjugating enzyme activity / Signaling by ALK fusions and activated point mutants /  regulation of cytokinesis / negative regulation of extrinsic apoptotic signaling pathway ...spongiotrophoblast layer development / labyrinthine layer development / ALK mutants bind TKIs / Flemming body / regulation of cytokinesis / negative regulation of extrinsic apoptotic signaling pathway ...spongiotrophoblast layer development / labyrinthine layer development / ALK mutants bind TKIs / Flemming body /  microtubule organizing center / cysteine-type endopeptidase inhibitor activity / ubiquitin conjugating enzyme activity / Signaling by ALK fusions and activated point mutants / microtubule organizing center / cysteine-type endopeptidase inhibitor activity / ubiquitin conjugating enzyme activity / Signaling by ALK fusions and activated point mutants /  regulation of cytokinesis / negative regulation of extrinsic apoptotic signaling pathway / RING-type E3 ubiquitin transferase / regulation of cytokinesis / negative regulation of extrinsic apoptotic signaling pathway / RING-type E3 ubiquitin transferase /  trans-Golgi network / trans-Golgi network /  spindle pole / ubiquitin-protein transferase activity / regulation of cell population proliferation / midbody / cell population proliferation / protein ubiquitination / spindle pole / ubiquitin-protein transferase activity / regulation of cell population proliferation / midbody / cell population proliferation / protein ubiquitination /  endosome / endosome /  cell cycle / cell cycle /  cell division / cell division /  protein phosphorylation / protein phosphorylation /  centrosome / apoptotic process / positive regulation of cell population proliferation / negative regulation of apoptotic process / centrosome / apoptotic process / positive regulation of cell population proliferation / negative regulation of apoptotic process /  membrane / membrane /  nucleus / nucleus /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.3 Å cryo EM / Resolution: 3.3 Å | ||||||
 Authors Authors | Dietz, L. / Elliott, P.R. | ||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| ||||||
 Citation Citation |  Journal: Science / Year: 2023 Journal: Science / Year: 2023Title: Structural basis for SMAC-mediated antagonism of caspase inhibition by the giant ubiquitin ligase BIRC6. Authors: Larissa Dietz / Cara J Ellison / Carlos Riechmann / C Keith Cassidy / F Daniel Felfoldi / Adán Pinto-Fernández / Benedikt M Kessler / Paul R Elliott /  Abstract: Certain inhibitor of apoptosis (IAP) family members are sentinel proteins that prevent untimely cell death by inhibiting caspases. Antagonists, including second mitochondria-derived activator of ...Certain inhibitor of apoptosis (IAP) family members are sentinel proteins that prevent untimely cell death by inhibiting caspases. Antagonists, including second mitochondria-derived activator of caspases (SMAC), regulate IAPs and drive cell death. Baculoviral IAP repeat-containing protein 6 (BIRC6), a giant IAP with dual E2 and E3 ubiquitin ligase activity, regulates programmed cell death through unknown mechanisms. We show that BIRC6 directly restricts executioner caspase-3 and -7 and ubiquitinates caspase-3, -7, and -9, working exclusively with noncanonical E1, UBA6. Notably, we show that SMAC suppresses both mechanisms. Cryo-electron microscopy structures of BIRC6 alone and in complex with SMAC reveal that BIRC6 is an antiparallel dimer juxtaposing the substrate-binding module against the catalytic domain. Furthermore, we discover that SMAC multisite binding to BIRC6 results in a subnanomolar affinity interaction, enabling SMAC to competitively displace caspases, thus antagonizing BIRC6 anticaspase function. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8atm.cif.gz 8atm.cif.gz | 774.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8atm.ent.gz pdb8atm.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  8atm.json.gz 8atm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/at/8atm https://data.pdbj.org/pub/pdb/validation_reports/at/8atm ftp://data.pdbj.org/pub/pdb/validation_reports/at/8atm ftp://data.pdbj.org/pub/pdb/validation_reports/at/8atm | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  15653MC  8atoC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 530977.000 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: BIRC6, KIAA1289 / Production host: Homo sapiens (human) / Gene: BIRC6, KIAA1289 / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm)References: UniProt: Q9NR09, RING-type E3 ubiquitin transferase |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Anti-parallel homodimer / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Molecular weight | Value: 1 MDa / Experimental value: YES |
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 4 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Instrument: FEI VITROBOT MARK III / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 105000 X / Nominal defocus max: 2500 nm / Nominal defocus min: 1000 nm / Calibrated defocus min: 1000 nm / Calibrated defocus max: 2500 nm / Cs Bright-field microscopy / Nominal magnification: 105000 X / Nominal defocus max: 2500 nm / Nominal defocus min: 1000 nm / Calibrated defocus min: 1000 nm / Calibrated defocus max: 2500 nm / Cs : 2.7 mm / C2 aperture diameter: 50 µm : 2.7 mm / C2 aperture diameter: 50 µm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 3.5 sec. / Electron dose: 49.9 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| EM software |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | |||||||||||||||
| Particle selection | Num. of particles selected: 548509 | |||||||||||||||
3D reconstruction | Resolution: 3.3 Å / Resolution method: OTHER / Num. of particles: 127710 Details: Derived from a composite map. See individual multibody maps for resolution. Symmetry type: POINT | |||||||||||||||
| Atomic model building | Protocol: OTHER / Space: REAL |
 Movie
Movie Controller
Controller






 PDBj
PDBj


