[English] 日本語
 Yorodumi
Yorodumi- PDB-8ah4: Crystal Structure of the third PDZ domain of PSD-95 protein in th... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8ah4 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the third PDZ domain of PSD-95 protein in the space group P3112 at pH 4.0 | ||||||
 Components Components | cDNA FLJ50577, highly similar to Discs large homolog 4 | ||||||
 Keywords Keywords |  PROTEIN BINDING / PROTEIN BINDING /  PDZ domain PDZ domain | ||||||
| Function / homology |  Function and homology information Function and homology information neuromuscular junction / neuromuscular junction /  kinase binding / chemical synaptic transmission / neuron projection kinase binding / chemical synaptic transmission / neuron projectionSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.48 Å molecular replacement / Resolution: 1.48 Å | ||||||
| Model details | pH 4.0 P3112 polymorph | ||||||
 Authors Authors | Camara-Artigas, A. / Salinas Garcia, M.C. | ||||||
| Funding support |  Spain, 1items Spain, 1items
| ||||||
 Citation Citation |  Journal: Crystals / Year: 2023 Journal: Crystals / Year: 2023Title: pH-Driven Polymorphic Behaviour of the Third PDZ Domain of PSD95: The Role of Electrostatic Interactions Authors: Salinas-Garcia, M.C. / Plaza-Garrido, M. / Gavira, J.A. / Murciano-Calles, J. / Andujar-Sanchez, M. / Ortiz-Salmeron, E. / Martinez, J.C. / Camara-Artigas, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8ah4.cif.gz 8ah4.cif.gz | 313.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8ah4.ent.gz pdb8ah4.ent.gz | 259.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8ah4.json.gz 8ah4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ah/8ah4 https://data.pdbj.org/pub/pdb/validation_reports/ah/8ah4 ftp://data.pdbj.org/pub/pdb/validation_reports/ah/8ah4 ftp://data.pdbj.org/pub/pdb/validation_reports/ah/8ah4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8ah5C  8ah6C  8ah7C  8ah8C  6qjiS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 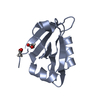
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components on special symmetry positions |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Ens-ID: 1
|
 Movie
Movie Controller
Controller


 PDBj
PDBj