+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7utp | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
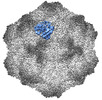
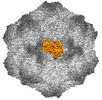
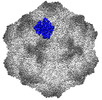
| Title | CPV Affinity Purified Polyclonal Fab A Site Fab | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | VIRUS/IMMUNE SYSTEM / CPV / polyclonal Fab /  A site / A site /  vaccination / vaccination /  VIRUS / VIRUS-IMMUNE SYSTEM complex VIRUS / VIRUS-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpermeabilization of host organelle membrane involved in viral entry into host cell / symbiont entry into host cell via permeabilization of inner membrane / microtubule-dependent intracellular transport of viral material towards nucleus / adhesion receptor-mediated virion attachment to host cell / T=1 icosahedral viral capsid / viral penetration into host nucleus / clathrin-dependent endocytosis of virus by host cell / entry receptor-mediated virion attachment to host cell / host cell nucleus / structural molecule activity /  metal ion binding metal ion bindingSimilarity search - Function | |||||||||
| Biological species |   Canis lupus familiaris (dog) Canis lupus familiaris (dog) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.8 Å cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Hartmann, S.R. / Hafenstein, S.L. / Charnesky, A.J. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2023 Journal: Commun Biol / Year: 2023Title: Cryo EM structures map a post vaccination polyclonal antibody response to canine parvovirus. Authors: Samantha R Hartmann / Andrew J Charnesky / Simon P Früh / Robert A López-Astacio / Wendy S Weichert / Nadia DiNunno / Sung Hung Cho / Carol M Bator / Colin R Parrish / Susan L Hafenstein /  Abstract: Canine parvovirus (CPV) is an important pathogen that emerged by cross-species transmission to cause severe disease in dogs. To understand the host immune response to vaccination, sera from dogs ...Canine parvovirus (CPV) is an important pathogen that emerged by cross-species transmission to cause severe disease in dogs. To understand the host immune response to vaccination, sera from dogs immunized with parvovirus are obtained, the polyclonal antibodies are purified and used to solve the high resolution cryo EM structures of the polyclonal Fab-virus complexes. We use a custom software, Icosahedral Subparticle Extraction and Correlated Classification (ISECC) to perform subparticle analysis and reconstruct polyclonal Fab-virus complexes from two different dogs eight and twelve weeks post vaccination. In the resulting polyclonal Fab-virus complexes there are a total of five distinct Fabs identified. In both cases, any of the five antibodies identified would interfere with receptor binding. This polyclonal mapping approach identifies a specific, limited immune response to the live vaccine virus and allows us to investigate the binding of multiple different antibodies or ligands to virus capsids. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7utp.cif.gz 7utp.cif.gz | 668.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7utp.ent.gz pdb7utp.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7utp.json.gz 7utp.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ut/7utp https://data.pdbj.org/pub/pdb/validation_reports/ut/7utp ftp://data.pdbj.org/pub/pdb/validation_reports/ut/7utp ftp://data.pdbj.org/pub/pdb/validation_reports/ut/7utp | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 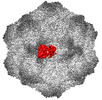 26786MC  7utrC  7utsC  7utuC  7utvC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein |  / Coat protein VP1 / Coat protein VP1Mass: 61562.367 Da / Num. of mol.: 8 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Canis lupus familiaris (dog) / Cell (production host): NLFK / Production host: Canis lupus familiaris (dog) / Cell (production host): NLFK / Production host:   Felis catus (domestic cat) / References: UniProt: Q11213 Felis catus (domestic cat) / References: UniProt: Q11213#2: Antibody | | Mass: 8443.399 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Canis lupus familiaris (dog) Canis lupus familiaris (dog)#3: Antibody | | Mass: 9209.344 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Canis lupus familiaris (dog) Canis lupus familiaris (dog)Sequence details | The authors state that the sequences of the antibody fragments are unknown. | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Canine parvovirus in complex with polyclonal fab / Type: COMPLEX / Entity ID: all / Source: MULTIPLE SOURCES / Type: COMPLEX / Entity ID: all / Source: MULTIPLE SOURCES |
|---|---|
| Source (natural) | Organism:   Canis lupus familiaris (dog) Canis lupus familiaris (dog) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2500 nm / Nominal defocus min: 500 nm Bright-field microscopy / Nominal defocus max: 2500 nm / Nominal defocus min: 500 nm |
| Image recording | Electron dose: 45 e/Å2 / Film or detector model: FEI FALCON III (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.20.1-4487_4487: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
3D reconstruction | Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 11073540 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj




