[English] 日本語
 Yorodumi
Yorodumi- PDB-7sil: Structure of positive allosteric modulator-bound active human cal... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7sil | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of positive allosteric modulator-bound active human calcium-sensing receptor | |||||||||||||||||||||
 Components Components | Isoform 1 of Extracellular calcium-sensing receptor | |||||||||||||||||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  calcium-sensing receptor / cryo-EM structure / calcium-sensing receptor / cryo-EM structure /  allosteric modulation / activation mechanism / allosteric modulation / activation mechanism /  symmetry symmetry | |||||||||||||||||||||
| Function / homology | Chem-9IG /  CHOLESTEROL / CHOLESTEROL /  PHOSPHATE ION / CYCLOMETHYLTRYPTOPHAN / Isoform 1 of Extracellular calcium-sensing receptor PHOSPHATE ION / CYCLOMETHYLTRYPTOPHAN / Isoform 1 of Extracellular calcium-sensing receptor Function and homology information Function and homology information | |||||||||||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.7 Å cryo EM / Resolution: 2.7 Å | |||||||||||||||||||||
 Authors Authors | Park, J. / Zuo, H. / Frangaj, A. / Fu, Z. / Yen, L.Y. / Zhang, Z. / Mosyak, L. / Slavkovich, V.N. / Liu, J. / Ray, K.M. ...Park, J. / Zuo, H. / Frangaj, A. / Fu, Z. / Yen, L.Y. / Zhang, Z. / Mosyak, L. / Slavkovich, V.N. / Liu, J. / Ray, K.M. / Cao, B. / Vallese, F. / Geng, Y. / Chen, S. / Grassucci, R. / Dandey, V.P. / Tan, Y.Z. / Eng, E. / Lee, Y. / Kloss, B. / Liu, Z. / Hendrickson, W.A. / Potter, C.S. / Carragher, B. / Graziano, J. / Conigrave, A.D. / Frank, J. / Clarke, O.B. / Fan, Q.R. | |||||||||||||||||||||
| Funding support |  United States, 6items United States, 6items
| |||||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: Symmetric activation and modulation of the human calcium-sensing receptor. Authors: Jinseo Park / Hao Zuo / Aurel Frangaj / Ziao Fu / Laura Y Yen / Zhening Zhang / Lidia Mosyak / Vesna N Slavkovich / Jonathan Liu / Kimberly M Ray / Baohua Cao / Francesca Vallese / Yong Geng ...Authors: Jinseo Park / Hao Zuo / Aurel Frangaj / Ziao Fu / Laura Y Yen / Zhening Zhang / Lidia Mosyak / Vesna N Slavkovich / Jonathan Liu / Kimberly M Ray / Baohua Cao / Francesca Vallese / Yong Geng / Shaoxia Chen / Robert Grassucci / Venkata P Dandey / Yong Zi Tan / Edward Eng / Yeji Lee / Brian Kloss / Zheng Liu / Wayne A Hendrickson / Clinton S Potter / Bridget Carragher / Joseph Graziano / Arthur D Conigrave / Joachim Frank / Oliver B Clarke / Qing R Fan /     Abstract: The human extracellular calcium-sensing (CaS) receptor controls plasma Ca levels and contributes to nutrient-dependent maintenance and metabolism of diverse organs. Allosteric modulation of the CaS ...The human extracellular calcium-sensing (CaS) receptor controls plasma Ca levels and contributes to nutrient-dependent maintenance and metabolism of diverse organs. Allosteric modulation of the CaS receptor corrects disorders of calcium homeostasis. Here, we report the cryogenic-electron microscopy reconstructions of a near-full-length CaS receptor in the absence and presence of allosteric modulators. Activation of the homodimeric CaS receptor requires a break in the transmembrane 6 (TM6) helix of each subunit, which facilitates the formation of a TM6-mediated homodimer interface and expansion of homodimer interactions. This transformation in TM6 occurs without a positive allosteric modulator. Two modulators with opposite functional roles bind to overlapping sites within the transmembrane domain through common interactions, acting to stabilize distinct rotamer conformations of key residues on the TM6 helix. The positive modulator reinforces TM6 distortion and maximizes subunit contact to enhance receptor activity, while the negative modulator strengthens an intact TM6 to dampen receptor function. In both active and inactive states, the receptor displays symmetrical transmembrane conformations that are consistent with its homodimeric assembly. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7sil.cif.gz 7sil.cif.gz | 288.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7sil.ent.gz pdb7sil.ent.gz | 242.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7sil.json.gz 7sil.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/si/7sil https://data.pdbj.org/pub/pdb/validation_reports/si/7sil ftp://data.pdbj.org/pub/pdb/validation_reports/si/7sil ftp://data.pdbj.org/pub/pdb/validation_reports/si/7sil | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  25143MC  7simC  7sinC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10834 (Title: Structures of positive allosteric modulator-bound and unbound active human calcium-sensing receptor EMPIAR-10834 (Title: Structures of positive allosteric modulator-bound and unbound active human calcium-sensing receptorData size: 3.8 TB Data #1: Unaligned multi-frame micrographs of calcium-sensing receptor in both PAM-bound and unbound active states [micrographs - multiframe]) |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 99299.461 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: CASR, GPRC2A, PCAR1 / Cell line (production host): HEK293 GnTl- / Production host: Homo sapiens (human) / Gene: CASR, GPRC2A, PCAR1 / Cell line (production host): HEK293 GnTl- / Production host:   Homo sapiens (human) / References: UniProt: P41180-1 Homo sapiens (human) / References: UniProt: P41180-1 |
|---|
-Sugars , 2 types, 10 molecules 
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose  / Mass: 424.401 Da / Num. of mol.: 4 / Mass: 424.401 Da / Num. of mol.: 4Source method: isolated from a genetically manipulated source #3: Sugar | ChemComp-NAG /  N-Acetylglucosamine N-Acetylglucosamine |
|---|
-Non-polymers , 5 types, 22 molecules 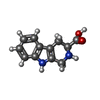








| #4: Chemical | | #5: Chemical |  Phosphate Phosphate#6: Chemical | ChemComp-CA / #7: Chemical | #8: Chemical | ChemComp-CLR /  Cholesterol Cholesterol |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Homodimer human calcium sensing receptor / Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.192 MDa / Experimental value: NO | ||||||||||||||||||||||||||||||||||||||||
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||||||||||||||||||
| Source (recombinant) | Organism:   Homo sapiens (human) / Cell: HEK 293 GnTl- / Plasmid Homo sapiens (human) / Cell: HEK 293 GnTl- / Plasmid : modified bacMam : modified bacMam | ||||||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 Details: Solution were made fresh from concentrated, and filtered to avoid microbial contamination. | ||||||||||||||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 2.6 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES / Details: This sample was monodisperse : YES / Details: This sample was monodisperse | ||||||||||||||||||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R0.6/1 | ||||||||||||||||||||||||||||||||||||||||
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K / Details: blot for 4 seconds before plunging |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS / Details: Preliminary grid screening was performed manually. |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 105000 X / Nominal defocus max: 2500 nm / Nominal defocus min: 1000 nm / Cs Bright-field microscopy / Nominal magnification: 105000 X / Nominal defocus max: 2500 nm / Nominal defocus min: 1000 nm / Cs : 2.7 mm / C2 aperture diameter: 70 µm / Alignment procedure: COMA FREE : 2.7 mm / C2 aperture diameter: 70 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Temperature (max): 100 K |
| Image recording | Electron dose: 70.35 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 13246 |
| EM imaging optics | Energyfilter name : GIF Quantum ER / Energyfilter slit width: 20 eV : GIF Quantum ER / Energyfilter slit width: 20 eV |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.19.1_4122: / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 892000 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C2 (2 fold cyclic : C2 (2 fold cyclic ) ) | ||||||||||||||||||||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 130000 / Algorithm: FOURIER SPACE / Details: Global(ECD) 2.7A TM 3.4A / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj












