+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7nip | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
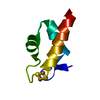
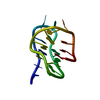
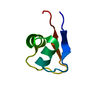
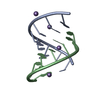
| Title | titin N2A unique sequence (UN2A) core | |||||||||
 Components Components | Isoform 11 of Titin | |||||||||
 Keywords Keywords |  STRUCTURAL PROTEIN / STRUCTURAL PROTEIN /  titin / N2A / UN2A titin / N2A / UN2A | |||||||||
| Function / homology |  Function and homology information Function and homology informationsarcomerogenesis / structural molecule activity conferring elasticity /  telethonin binding / skeletal muscle myosin thick filament assembly / cardiac myofibril assembly / muscle alpha-actinin binding / detection of muscle stretch / cardiac muscle tissue morphogenesis / telethonin binding / skeletal muscle myosin thick filament assembly / cardiac myofibril assembly / muscle alpha-actinin binding / detection of muscle stretch / cardiac muscle tissue morphogenesis /  regulation of catalytic activity / cardiac muscle hypertrophy ...sarcomerogenesis / structural molecule activity conferring elasticity / regulation of catalytic activity / cardiac muscle hypertrophy ...sarcomerogenesis / structural molecule activity conferring elasticity /  telethonin binding / skeletal muscle myosin thick filament assembly / cardiac myofibril assembly / muscle alpha-actinin binding / detection of muscle stretch / cardiac muscle tissue morphogenesis / telethonin binding / skeletal muscle myosin thick filament assembly / cardiac myofibril assembly / muscle alpha-actinin binding / detection of muscle stretch / cardiac muscle tissue morphogenesis /  regulation of catalytic activity / cardiac muscle hypertrophy / mitotic chromosome condensation / Striated Muscle Contraction / regulation of catalytic activity / cardiac muscle hypertrophy / mitotic chromosome condensation / Striated Muscle Contraction /  M band / M band /  actinin binding / I band / cardiac muscle cell development / actinin binding / I band / cardiac muscle cell development /  regulation of protein kinase activity / sarcomere organization / structural constituent of muscle / skeletal muscle thin filament assembly / striated muscle thin filament / striated muscle contraction / protein kinase A signaling / cardiac muscle contraction / regulation of protein kinase activity / sarcomere organization / structural constituent of muscle / skeletal muscle thin filament assembly / striated muscle thin filament / striated muscle contraction / protein kinase A signaling / cardiac muscle contraction /  muscle contraction / condensed nuclear chromosome / positive regulation of protein secretion / Z disc / response to calcium ion / muscle contraction / condensed nuclear chromosome / positive regulation of protein secretion / Z disc / response to calcium ion /  : / : /  actin filament binding / Platelet degranulation / actin filament binding / Platelet degranulation /  protein tyrosine kinase activity / protein tyrosine kinase activity /  protease binding / protease binding /  calmodulin binding / calmodulin binding /  non-specific serine/threonine protein kinase / non-specific serine/threonine protein kinase /  phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity /  calcium ion binding / positive regulation of gene expression / calcium ion binding / positive regulation of gene expression /  protein kinase binding / protein kinase binding /  enzyme binding / extracellular exosome / extracellular region / enzyme binding / extracellular exosome / extracellular region /  ATP binding / identical protein binding / ATP binding / identical protein binding /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  SOLUTION NMR / na SOLUTION NMR / na | |||||||||
 Authors Authors | Zhou, T. / Kovermann, M. / Fleming, J.R. / Mayans, O. | |||||||||
| Funding support |  France, European Union, 2items France, European Union, 2items
| |||||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2021 Journal: J.Mol.Biol. / Year: 2021Title: Molecular Characterisation of Titin N2A and Its Binding of CARP Reveals a Titin/Actin Cross-linking Mechanism. Authors: Zhou, T. / Fleming, J.R. / Lange, S. / Hessel, A.L. / Bogomolovas, J. / Stronczek, C. / Grundei, D. / Ghassemian, M. / Biju, A. / Borgeson, E. / Bullard, B. / Linke, W.A. / Chen, J. / Kovermann, M. / Mayans, O. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7nip.cif.gz 7nip.cif.gz | 133.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7nip.ent.gz pdb7nip.ent.gz | 114.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7nip.json.gz 7nip.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ni/7nip https://data.pdbj.org/pub/pdb/validation_reports/ni/7nip ftp://data.pdbj.org/pub/pdb/validation_reports/ni/7nip ftp://data.pdbj.org/pub/pdb/validation_reports/ni/7nip | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein/peptide | Mass: 4932.733 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: TTN / Production host: Homo sapiens (human) / Gene: TTN / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: Q8WZ42,  non-specific serine/threonine protein kinase non-specific serine/threonine protein kinase |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
|
- Sample preparation
Sample preparation
| Details | Type: solution Contents: 30 mM sodium phosphate, 100 mM sodium chloride, 95% H2O/5% D2O Label: sample_un2a / Solvent system: 95% H2O/5% D2O | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample |
| ||||||||||||
| Sample conditions | Ionic strength: 100 mM / Label: conditions_1 / pH: 7.5 / Pressure: 101300 Pa / Temperature: 298 K |
-NMR measurement
| NMR spectrometer | Type: Bruker AVANCE III / Manufacturer: Bruker / Model : AVANCE III / Field strength: 600 MHz : AVANCE III / Field strength: 600 MHz |
|---|
- Processing
Processing
| NMR software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: na / Software ordinal: 1 | ||||||||||||||||
| NMR representative | Selection criteria: closest to the average | ||||||||||||||||
| NMR ensemble | Conformer selection criteria: all calculated structures submitted Conformers calculated total number: 32500 / Conformers submitted total number: 10 |
 Movie
Movie Controller
Controller







 PDBj
PDBj



