+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7l3m | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | PEPCK MMQX structure 40ms post-mixing with oxaloacetic acid | |||||||||
 Components Components | Phosphoenolpyruvate carboxykinase, cytosolic [GTP] | |||||||||
 Keywords Keywords |  LYASE / LYASE /  PEPCK / PEPCK /  phosphoenolpyruvate carboxykinase / gluconeogensis phosphoenolpyruvate carboxykinase / gluconeogensis | |||||||||
| Function / homology |  Function and homology information Function and homology information Gluconeogenesis / Gluconeogenesis /  phosphoenolpyruvate carboxykinase activity / cellular response to potassium ion starvation / protein serine kinase activity (using GTP as donor) / response to methionine / glycerol biosynthetic process from pyruvate / phosphoenolpyruvate carboxykinase activity / cellular response to potassium ion starvation / protein serine kinase activity (using GTP as donor) / response to methionine / glycerol biosynthetic process from pyruvate /  Transferases; Transferring phosphorus-containing groups; Protein-serine/threonine kinases / Transferases; Transferring phosphorus-containing groups; Protein-serine/threonine kinases /  phosphoenolpyruvate carboxykinase (GTP) / phosphoenolpyruvate carboxykinase (GTP) /  phosphoenolpyruvate carboxykinase (GTP) activity / propionate catabolic process ... phosphoenolpyruvate carboxykinase (GTP) activity / propionate catabolic process ... Gluconeogenesis / Gluconeogenesis /  phosphoenolpyruvate carboxykinase activity / cellular response to potassium ion starvation / protein serine kinase activity (using GTP as donor) / response to methionine / glycerol biosynthetic process from pyruvate / phosphoenolpyruvate carboxykinase activity / cellular response to potassium ion starvation / protein serine kinase activity (using GTP as donor) / response to methionine / glycerol biosynthetic process from pyruvate /  Transferases; Transferring phosphorus-containing groups; Protein-serine/threonine kinases / Transferases; Transferring phosphorus-containing groups; Protein-serine/threonine kinases /  phosphoenolpyruvate carboxykinase (GTP) / phosphoenolpyruvate carboxykinase (GTP) /  phosphoenolpyruvate carboxykinase (GTP) activity / propionate catabolic process / cellular response to raffinose / tricarboxylic acid metabolic process / regulation of lipid biosynthetic process / response to interleukin-6 / cellular response to fructose stimulus / cellular hypotonic response / glyceraldehyde-3-phosphate biosynthetic process / phosphoenolpyruvate carboxykinase (GTP) activity / propionate catabolic process / cellular response to raffinose / tricarboxylic acid metabolic process / regulation of lipid biosynthetic process / response to interleukin-6 / cellular response to fructose stimulus / cellular hypotonic response / glyceraldehyde-3-phosphate biosynthetic process /  carboxylic acid binding / cellular hypotonic salinity response / cellular response to phorbol 13-acetate 12-myristate / oxaloacetate metabolic process / hepatocyte differentiation / cellular hyperosmotic response / positive regulation of memory T cell differentiation / carboxylic acid binding / cellular hypotonic salinity response / cellular response to phorbol 13-acetate 12-myristate / oxaloacetate metabolic process / hepatocyte differentiation / cellular hyperosmotic response / positive regulation of memory T cell differentiation /  nucleoside diphosphate kinase activity / cellular hyperosmotic salinity response / response to lipid / response to starvation / cellular response to glucagon stimulus / cellular response to interleukin-1 / positive regulation of lipid biosynthetic process / cellular response to retinoic acid / cellular response to cAMP / cellular response to dexamethasone stimulus / response to nutrient levels / response to activity / nucleoside diphosphate kinase activity / cellular hyperosmotic salinity response / response to lipid / response to starvation / cellular response to glucagon stimulus / cellular response to interleukin-1 / positive regulation of lipid biosynthetic process / cellular response to retinoic acid / cellular response to cAMP / cellular response to dexamethasone stimulus / response to nutrient levels / response to activity /  gluconeogenesis / cellular response to glucose stimulus / response to bacterium / response to insulin / lipid metabolic process / cellular response to insulin stimulus / glucose metabolic process / GDP binding / gluconeogenesis / cellular response to glucose stimulus / response to bacterium / response to insulin / lipid metabolic process / cellular response to insulin stimulus / glucose metabolic process / GDP binding /  glucose homeostasis / manganese ion binding / cellular response to tumor necrosis factor / cellular response to hypoxia / peptidyl-serine phosphorylation / response to lipopolysaccharide / GTP binding / magnesium ion binding / glucose homeostasis / manganese ion binding / cellular response to tumor necrosis factor / cellular response to hypoxia / peptidyl-serine phosphorylation / response to lipopolysaccharide / GTP binding / magnesium ion binding /  endoplasmic reticulum / positive regulation of transcription by RNA polymerase II / endoplasmic reticulum / positive regulation of transcription by RNA polymerase II /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.07 Å FOURIER SYNTHESIS / Resolution: 2.07 Å | |||||||||
 Authors Authors | Clinger, J.A. / Moreau, D.W. / McLeod, M.J. / Holyoak, T. / Thorne, R.E. | |||||||||
| Funding support |  United States, United States,  Canada, 2items Canada, 2items
| |||||||||
 Citation Citation |  Journal: Iucrj / Year: 2021 Journal: Iucrj / Year: 2021Title: Millisecond mix-and-quench crystallography (MMQX) enables time-resolved studies of PEPCK with remote data collection. Authors: Clinger, J.A. / Moreau, D.W. / McLeod, M.J. / Holyoak, T. / Thorne, R.E. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7l3m.cif.gz 7l3m.cif.gz | 305.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7l3m.ent.gz pdb7l3m.ent.gz | 213.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7l3m.json.gz 7l3m.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/l3/7l3m https://data.pdbj.org/pub/pdb/validation_reports/l3/7l3m ftp://data.pdbj.org/pub/pdb/validation_reports/l3/7l3m ftp://data.pdbj.org/pub/pdb/validation_reports/l3/7l3m | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7l36C  7l3vC  6p5oS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein |  / PEPCK-C / PEPCK-CMass: 69643.789 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Pck1 / Plasmid: PGEX4T2 / Production host: Rattus norvegicus (Norway rat) / Gene: Pck1 / Plasmid: PGEX4T2 / Production host:   Escherichia coli BL21(DE3) (bacteria) Escherichia coli BL21(DE3) (bacteria)References: UniProt: P07379,  phosphoenolpyruvate carboxykinase (GTP), phosphoenolpyruvate carboxykinase (GTP),  Transferases; Transferring phosphorus-containing groups; Protein-serine/threonine kinases Transferases; Transferring phosphorus-containing groups; Protein-serine/threonine kinases |
|---|
-Non-polymers , 6 types, 326 molecules 

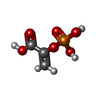

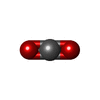






| #2: Chemical | | #3: Chemical | ChemComp-NA / | #4: Chemical | ChemComp-PEP / |  Phosphoenolpyruvic acid Phosphoenolpyruvic acid#5: Chemical | ChemComp-GDP / |  Guanosine diphosphate Guanosine diphosphate#6: Chemical | ChemComp-CO2 / |  Carbon dioxide Carbon dioxide#7: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.16 Å3/Da / Density % sol: 43.03 % / Mosaicity: 0 ° |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion / pH: 7.5 / Details: 25mM MnCl2, 25mM GTP, 19% PEG 3350, 100mM HEPES |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CHESS CHESS  / Beamline: F1 / Wavelength: 1.12 Å / Beamline: F1 / Wavelength: 1.12 Å | ||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Feb 5, 2020 | ||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 1.12 Å / Relative weight: 1 : 1.12 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.07→59.47 Å / Num. obs: 35893 / % possible obs: 99.9 % / Redundancy: 3.6 % / CC1/2: 0.967 / Rmerge(I) obs: 0.168 / Rpim(I) all: 0.103 / Rrim(I) all: 0.198 / Net I/σ(I): 6.1 / Num. measured all: 127837 / Scaling rejects: 1078 | ||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: 6P5O Resolution: 2.07→51.33 Å / SU ML: 0.239 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 18.6799 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 23.64 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.07→51.33 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -2.16834553195 Å / Origin y: -0.234906753778 Å / Origin z: 7.74140547381 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller

















 PDBj
PDBj






