+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6zbk | ||||||
|---|---|---|---|---|---|---|---|
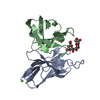
| Title | Crystal structure of the human complex between RPAP3 and TRBP | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  RNA BINDING PROTEIN / RNPs / MicroRNAs / RNA Induced Silencing Complex / dsRNA pathways RNA BINDING PROTEIN / RNPs / MicroRNAs / RNA Induced Silencing Complex / dsRNA pathways | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of siRNA processing / regulation of miRNA processing / regulation of viral transcription / negative regulation of cytoplasmic pattern recognition receptor signaling pathway / regulation of regulatory ncRNA processing / negative regulation of defense response to virus by host / global gene silencing by mRNA cleavage / pre-miRNA binding / Small interfering RNA (siRNA) biogenesis / R2TP complex ...regulation of siRNA processing / regulation of miRNA processing / regulation of viral transcription / negative regulation of cytoplasmic pattern recognition receptor signaling pathway / regulation of regulatory ncRNA processing / negative regulation of defense response to virus by host / global gene silencing by mRNA cleavage / pre-miRNA binding / Small interfering RNA (siRNA) biogenesis / R2TP complex / RPAP3/R2TP/prefoldin-like complex / RISC-loading complex / RISC complex assembly / miRNA processing / pre-miRNA processing / siRNA processing / siRNA binding /  pre-mRNA binding / RISC complex / protein folding chaperone complex / MicroRNA (miRNA) biogenesis / miRNA binding / positive regulation of viral genome replication / protein sequestering activity / negative regulation of protein kinase activity / PKR-mediated signaling / pre-mRNA binding / RISC complex / protein folding chaperone complex / MicroRNA (miRNA) biogenesis / miRNA binding / positive regulation of viral genome replication / protein sequestering activity / negative regulation of protein kinase activity / PKR-mediated signaling /  regulation of translation / regulation of translation /  double-stranded RNA binding / protein stabilization / double-stranded RNA binding / protein stabilization /  nuclear body / perinuclear region of cytoplasm / nuclear body / perinuclear region of cytoplasm /  enzyme binding / protein homodimerization activity / enzyme binding / protein homodimerization activity /  nucleoplasm / identical protein binding / nucleoplasm / identical protein binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.49 Å molecular replacement / Resolution: 1.49 Å | ||||||
 Authors Authors | Charron, C. / Abel, Y. / Charpentier, B. / Rederstorff, M. | ||||||
 Citation Citation |  Journal: Nucleic Acids Res. / Year: 2022 Journal: Nucleic Acids Res. / Year: 2022Title: The interaction between RPAP3 and TRBP reveals a possible involvement of the HSP90/R2TP chaperone complex in the regulation of miRNA activity. Authors: Abel, Y. / Charron, C. / Virciglio, C. / Bourguignon-Igel, V. / Quinternet, M. / Chagot, M.E. / Robert, M.C. / Verheggen, C. / Branlant, C. / Bertrand, E. / Manival, X. / Charpentier, B. / Rederstorff, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6zbk.cif.gz 6zbk.cif.gz | 62.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6zbk.ent.gz pdb6zbk.ent.gz | 43.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6zbk.json.gz 6zbk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zb/6zbk https://data.pdbj.org/pub/pdb/validation_reports/zb/6zbk ftp://data.pdbj.org/pub/pdb/validation_reports/zb/6zbk ftp://data.pdbj.org/pub/pdb/validation_reports/zb/6zbk | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 14066.897 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: RPAP3 / Production host: Homo sapiens (human) / Gene: RPAP3 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q9H6T3 Escherichia coli (E. coli) / References: UniProt: Q9H6T3 |
|---|---|
| #2: Protein | Mass: 11597.268 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: TARBP2, TRBP / Production host: Homo sapiens (human) / Gene: TARBP2, TRBP / Production host:   Escherichia coli (E. coli) / References: UniProt: Q15633 Escherichia coli (E. coli) / References: UniProt: Q15633 |
| #3: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2 Å3/Da / Density % sol: 38.6 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 6 / Details: PEG 3,350 and TacsimateTM |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 1.033 Å / Beamline: ID29 / Wavelength: 1.033 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: May 4, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.033 Å / Relative weight: 1 : 1.033 Å / Relative weight: 1 |
| Reflection | Resolution: 1.49→39.53 Å / Num. obs: 34245 / % possible obs: 99 % / Redundancy: 6.2 % / Rsym value: 0.059 / Net I/σ(I): 18.6 |
| Reflection shell | Resolution: 1.49→1.58 Å / Redundancy: 6.3 % / Mean I/σ(I) obs: 5.1 / Num. unique obs: 5166 / Rsym value: 0.279 / % possible all: 94.1 |
-Phasing
Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4CGV,4WYQ Resolution: 1.49→39.53 Å / Cor.coef. Fo:Fc: 0.964 / Cor.coef. Fo:Fc free: 0.951 / SU B: 1.407 / SU ML: 0.052 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.077 / ESU R Free: 0.08 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 53.03 Å2 / Biso mean: 16.14 Å2 / Biso min: 6.3 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.49→39.53 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.494→1.533 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller














 PDBj
PDBj






