[English] 日本語
 Yorodumi
Yorodumi- PDB-6gtv: Crystal structure of a FimH*DsG complex from E.coli F18 with boun... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6gtv | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of a FimH*DsG complex from E.coli F18 with bound trimannose | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords |  CELL ADHESION / TYPE I PILUS / CATCH-BOND / CELL ADHESION / TYPE I PILUS / CATCH-BOND /  LECTIN / UPEC / LECTIN / UPEC /  INFECTION / INFECTION /  MANNOSE MANNOSE | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |   Escherichia coli F18+ (bacteria) Escherichia coli F18+ (bacteria)  Escherichia coli 536 (bacteria) Escherichia coli 536 (bacteria) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | |||||||||
 Authors Authors | Jakob, R.P. / Sauer, M.M. / Luber, T. / Canonica, F. / Navarra, G. / Ernst, B. / Unverzagt, C. / Maier, T. / Glockshuber, R. | |||||||||
 Citation Citation |  Journal: J Am Chem Soc / Year: 2019 Journal: J Am Chem Soc / Year: 2019Title: Binding of the Bacterial Adhesin FimH to Its Natural, Multivalent High-Mannose Type Glycan Targets. Authors: Maximilian M Sauer / Roman P Jakob / Thomas Luber / Fabia Canonica / Giulio Navarra / Beat Ernst / Carlo Unverzagt / Timm Maier / Rudi Glockshuber /   Abstract: Multivalent carbohydrate-lectin interactions at host-pathogen interfaces play a crucial role in the establishment of infections. Although competitive antagonists that prevent pathogen adhesion are ...Multivalent carbohydrate-lectin interactions at host-pathogen interfaces play a crucial role in the establishment of infections. Although competitive antagonists that prevent pathogen adhesion are promising antimicrobial drugs, the molecular mechanisms underlying these complex adhesion processes are still poorly understood. Here, we characterize the interactions between the fimbrial adhesin FimH from uropathogenic Escherichia coli strains and its natural high-mannose type N-glycan binding epitopes on uroepithelial glycoproteins. Crystal structures and a detailed kinetic characterization of ligand-binding and dissociation revealed that the binding pocket of FimH evolved such that it recognizes the terminal α(1-2)-, α(1-3)-, and α(1-6)-linked mannosides of natural high-mannose type N-glycans with similar affinity. We demonstrate that the 2000-fold higher affinity of the domain-separated state of FimH compared to its domain-associated state is ligand-independent and consistent with a thermodynamic cycle in which ligand-binding shifts the association equilibrium between the FimH lectin and the FimH pilin domain. Moreover, we show that a single N-glycan can bind up to three molecules of FimH, albeit with negative cooperativity, so that a molar excess of accessible N-glycans over FimH on the cell surface favors monovalent FimH binding. Our data provide pivotal insights into the adhesion properties of uropathogenic Escherichia coli strains to their target receptors and a solid basis for the development of effective FimH antagonists. | |||||||||
| History |
|
- Structure visualization
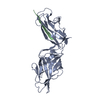
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6gtv.cif.gz 6gtv.cif.gz | 331.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6gtv.ent.gz pdb6gtv.ent.gz | 277.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6gtv.json.gz 6gtv.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gt/6gtv https://data.pdbj.org/pub/pdb/validation_reports/gt/6gtv ftp://data.pdbj.org/pub/pdb/validation_reports/gt/6gtv ftp://data.pdbj.org/pub/pdb/validation_reports/gt/6gtv | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6gtwC  6gtxC  6gtyC  6gtzC  6gu0C  4xoeS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Protein/peptide / Sugars , 3 types, 5 molecules ACBD
| #1: Protein | Mass: 29053.260 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli F18+ (bacteria) / Gene: ECP_4655, fimh / Plasmid: pTRC99a / Production host: Escherichia coli F18+ (bacteria) / Gene: ECP_4655, fimh / Plasmid: pTRC99a / Production host:   Escherichia coli (E. coli) / Strain (production host): HM125 / References: UniProt: A0A0R4I961 Escherichia coli (E. coli) / Strain (production host): HM125 / References: UniProt: A0A0R4I961#2: Protein/peptide | Mass: 1416.661 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.)   Escherichia coli 536 (bacteria) / References: UniProt: A0A140UH97 Escherichia coli 536 (bacteria) / References: UniProt: A0A140UH97#3: Polysaccharide | alpha-D-mannopyranose-(1-3)-[alpha-D-mannopyranose-(1-6)]alpha-D-mannopyranose |  / Mass: 504.438 Da / Num. of mol.: 1 / Mass: 504.438 Da / Num. of mol.: 1Source method: isolated from a genetically manipulated source |
|---|
-Non-polymers , 3 types, 463 molecules 




| #4: Chemical | ChemComp-PEG /  Diethylene glycol Diethylene glycol#5: Chemical | ChemComp-1PE / |  Polyethylene glycol Polyethylene glycol#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.44 Å3/Da / Density % sol: 64.26 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / Details: 0.2 M CaCl2, 0.1 M Hepes pH 7.5, 28% PEG 400 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 1.00001 Å / Beamline: X06SA / Wavelength: 1.00001 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Oct 21, 2013 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.00001 Å / Relative weight: 1 : 1.00001 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→48.727 Å / Num. obs: 49075 / % possible obs: 99.9 % / Redundancy: 6.6 % / CC1/2: 0.998 / Rrim(I) all: 0.106 / Net I/σ(I): 13 |
| Reflection shell | Resolution: 2.1→2.22 Å / Redundancy: 6.4 % / Mean I/σ(I) obs: 1.8 / Num. unique obs: 7428 / CC1/2: 0.729 / Rrim(I) all: 1.151 / % possible all: 99.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4XOE Resolution: 2.1→48.727 Å / SU ML: 0.26 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 22.55
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→48.727 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj

