[English] 日本語
 Yorodumi
Yorodumi- PDB-6ceu: MBD3 MBD in complex with methylated, non-palindromic CpG DNA: alt... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6ceu | ||||||
|---|---|---|---|---|---|---|---|
| Title | MBD3 MBD in complex with methylated, non-palindromic CpG DNA: alternative interpretation of crystallographic data | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION/DNA /  Structural Genomics Consortium / SGC / TRANSCRIPTION-DNA complex Structural Genomics Consortium / SGC / TRANSCRIPTION-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology informationtissue development / regulation of cell fate specification / : / DNA methylation-dependent heterochromatin formation / NuRD complex /  regulation of stem cell differentiation / methyl-CpG binding / RNA Polymerase I Transcription Initiation / embryonic organ development / regulation of stem cell differentiation / methyl-CpG binding / RNA Polymerase I Transcription Initiation / embryonic organ development /  heterochromatin ...tissue development / regulation of cell fate specification / : / DNA methylation-dependent heterochromatin formation / NuRD complex / heterochromatin ...tissue development / regulation of cell fate specification / : / DNA methylation-dependent heterochromatin formation / NuRD complex /  regulation of stem cell differentiation / methyl-CpG binding / RNA Polymerase I Transcription Initiation / embryonic organ development / regulation of stem cell differentiation / methyl-CpG binding / RNA Polymerase I Transcription Initiation / embryonic organ development /  heterochromatin / Regulation of TP53 Activity through Acetylation / response to nutrient levels / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / Regulation of PTEN gene transcription / HDACs deacetylate histones / response to estradiol / heterochromatin / Regulation of TP53 Activity through Acetylation / response to nutrient levels / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / Regulation of PTEN gene transcription / HDACs deacetylate histones / response to estradiol /  heart development / in utero embryonic development / Potential therapeutics for SARS / heart development / in utero embryonic development / Potential therapeutics for SARS /  chromatin remodeling / negative regulation of DNA-templated transcription / chromatin remodeling / negative regulation of DNA-templated transcription /  chromatin binding / chromatin binding /  chromatin / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / protein-containing complex / chromatin / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / protein-containing complex /  DNA binding / DNA binding /  nucleoplasm / nucleoplasm /  nucleus / nucleus /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.005 Å FOURIER SYNTHESIS / Resolution: 2.005 Å | ||||||
 Authors Authors | Liu, K. / Tempel, W. / Wernimont, A.K. / Bountra, C. / Arrowsmith, C.H. / Edwards, A.M. / Min, J. / Structural Genomics Consortium (SGC) | ||||||
 Citation Citation |  Journal: Febs J. / Year: 2019 Journal: Febs J. / Year: 2019Title: Structural analyses reveal that MBD3 is a methylated CG binder. Authors: Liu, K. / Lei, M. / Wu, Z. / Gan, B. / Cheng, H. / Li, Y. / Min, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6ceu.cif.gz 6ceu.cif.gz | 124.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6ceu.ent.gz pdb6ceu.ent.gz | 92.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6ceu.json.gz 6ceu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ce/6ceu https://data.pdbj.org/pub/pdb/validation_reports/ce/6ceu ftp://data.pdbj.org/pub/pdb/validation_reports/ce/6ceu ftp://data.pdbj.org/pub/pdb/validation_reports/ce/6ceu | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6cc8SC  6ccgC  6cevC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 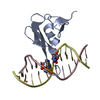
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein |  / Methyl-CpG-binding protein MBD3 / Methyl-CpG-binding protein MBD3Mass: 8473.716 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: MBD3 / Plasmid: PET28-GSTLIC / Production host: Homo sapiens (human) / Gene: MBD3 / Plasmid: PET28-GSTLIC / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21-V2R-pRARE2 / References: UniProt: O95983 Escherichia coli (E. coli) / Strain (production host): BL21-V2R-pRARE2 / References: UniProt: O95983#2: DNA chain | Mass: 3693.418 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) #3: DNA chain | Mass: 3662.408 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) #4: Chemical | ChemComp-UNX / #5: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.8 Å3/Da / Density % sol: 55.5 % |
|---|---|
Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 6.5 / Details: 25% PEG3350, 0.2M sodium chloride, 0.1M bis-tris |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.97911 Å / Beamline: 19-ID / Wavelength: 0.97911 Å | ||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Dec 14, 2012 | ||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 0.97911 Å / Relative weight: 1 : 0.97911 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||
| Reflection | Resolution: 2→43.69 Å / Num. obs: 23289 / % possible obs: 99.6 % / Redundancy: 3.6 % / Biso Wilson estimate: 40.95 Å2 / CC1/2: 0.996 / Rmerge(I) obs: 0.069 / Rpim(I) all: 0.043 / Rrim(I) all: 0.081 / Net I/σ(I): 11.1 / Num. measured all: 84001 / Scaling rejects: 0 | ||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: early version of model from PDB entry 6CC8 Resolution: 2.005→35.202 Å / SU ML: 0.29 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 26.78 Details: Electron density did not permit unambiguous distinction between strands of non-palindromic DNA. refmac was used for intermediate refinement steps. coot was used for interactive model ...Details: Electron density did not permit unambiguous distinction between strands of non-palindromic DNA. refmac was used for intermediate refinement steps. coot was used for interactive model building. Model geometry was validated with molprobity.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 95.22 Å2 / Biso mean: 43.1122 Å2 / Biso min: 24.87 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.005→35.202 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 9
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj












































