[English] 日本語
 Yorodumi
Yorodumi- PDB-5ycw: Double domain swapped dimer of engineered hairpin loop1 and loop3... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ycw | ||||||
|---|---|---|---|---|---|---|---|
| Title | Double domain swapped dimer of engineered hairpin loop1 and loop3 mutant in Single-chain Monellin | ||||||
 Components Components | single chain monellin | ||||||
 Keywords Keywords |  PLANT PROTEIN / domain swapped dimer / Single-chain Monellin / loop mutation / QVVAG motif PLANT PROTEIN / domain swapped dimer / Single-chain Monellin / loop mutation / QVVAG motif | ||||||
| Function / homology |  Monellin, B chain / Monellin, B chain /  Monellin / Monellin /  Monellin / Cystatin superfamily / Monellin chain B Monellin / Cystatin superfamily / Monellin chain B Function and homology information Function and homology information | ||||||
| Biological species |   Dioscoreophyllum cumminsii (serendipity berry) Dioscoreophyllum cumminsii (serendipity berry) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.285 Å MOLECULAR REPLACEMENT / Resolution: 2.285 Å | ||||||
 Authors Authors | Surana, P. / Nandwani, N. / Udgaonkar, J.B. / Gosavi, S. / Das, R. | ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: A five-residue motif for the design of domain swapping in proteins. Authors: Nandwani, N. / Surana, P. / Negi, H. / Mascarenhas, N.M. / Udgaonkar, J.B. / Das, R. / Gosavi, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ycw.cif.gz 5ycw.cif.gz | 51.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ycw.ent.gz pdb5ycw.ent.gz | 35.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ycw.json.gz 5ycw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yc/5ycw https://data.pdbj.org/pub/pdb/validation_reports/yc/5ycw ftp://data.pdbj.org/pub/pdb/validation_reports/yc/5ycw ftp://data.pdbj.org/pub/pdb/validation_reports/yc/5ycw | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5yctC  5ycuC  6iwjC  1iv7S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 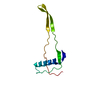
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 10622.219 Da / Num. of mol.: 1 Mutation: YENEGFREIK to QVVA in loop1, DYKTR to QVVAG in loop3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Dioscoreophyllum cumminsii (serendipity berry) Dioscoreophyllum cumminsii (serendipity berry)Production host:   Escherichia coli (E. coli) / References: UniProt: P02882*PLUS Escherichia coli (E. coli) / References: UniProt: P02882*PLUS |
|---|---|
| #2: Water | ChemComp-HOH /  Water Water |
| Sequence details | The complete sequence of single chain Monellin has been deposited to NCBI with accession code ...The complete sequence of single chain Monellin has been deposited to NCBI with accession code AFF58925. Residues 48-57 YENEGFREIK |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.25 Å3/Da / Density % sol: 62.21 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop Details: 8-12% (wt/vol) PEG 8000, 50mM sodium phosphate, pH 6.4-6.8, Crystals grew in a week PH range: 6.4-7.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: MASSIF-3 / Wavelength: 0.966 Å / Beamline: MASSIF-3 / Wavelength: 0.966 Å |
| Detector | Type: DECTRIS PILATUS3 2M / Detector: PIXEL / Date: Apr 20, 2017 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.966 Å / Relative weight: 1 : 0.966 Å / Relative weight: 1 |
| Reflection | Resolution: 2.285→41.66 Å / Num. obs: 6521 / % possible obs: 97.28 % / Redundancy: 5.9 % / Biso Wilson estimate: 71.49 Å2 / CC1/2: 0.999 / Rmerge(I) obs: 0.03691 / Net I/σ(I): 22.76 |
| Reflection shell | Highest resolution: 2.285 Å / Rmerge(I) obs: 0.7052 / Mean I/σ(I) obs: 2.46 / CC1/2: 0.85 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1IV7 Resolution: 2.285→41.66 Å / SU ML: 0.3 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 34.91
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.285→41.66 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj

