Entry Database : PDB / ID : 5w0eTitle CREBBP bromodomain in complex with Cpd19 (3-(7-(difluoromethyl)-6-(1-methyl-1H-pyrazol-4-yl)-3,4-dihydroquinolin-1(2H)-yl)-N-methyl-1-(tetrahydro-2H-pyran-4-yl)-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carboxamide) CREB-binding protein Keywords / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / / Resolution : 1.41 Å Authors Murray, J.M. Journal : J. Med. Chem. / Year : 2017Title : A Unique Approach to Design Potent and Selective Cyclic Adenosine Monophosphate Response Element Binding Protein, Binding Protein (CBP) Inhibitors.Authors: Bronner, S.M. / Murray, J. / Romero, F.A. / Lai, K.W. / Tsui, V. / Cyr, P. / Beresini, M.H. / de Leon Boenig, G. / Chen, Z. / Choo, E.F. / Clark, K.R. / Crawford, T.D. / Jayaram, H. / ... Authors : Bronner, S.M. / Murray, J. / Romero, F.A. / Lai, K.W. / Tsui, V. / Cyr, P. / Beresini, M.H. / de Leon Boenig, G. / Chen, Z. / Choo, E.F. / Clark, K.R. / Crawford, T.D. / Jayaram, H. / Kaufman, S. / Li, R. / Li, Y. / Liao, J. / Liang, X. / Liu, W. / Ly, J. / Maher, J. / Wai, J. / Wang, F. / Zheng, A. / Zhu, X. / Magnuson, S. History Deposition May 30, 2017 Deposition site / Processing site Revision 1.0 Feb 21, 2018 Provider / Type Revision 1.1 Oct 4, 2023 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components
 Keywords
Keywords CREBBP /
CREBBP /  Bromodomain / small molecule inhibitor / TRANSCRIPTION REGULATOR-INHIBITOR complex
Bromodomain / small molecule inhibitor / TRANSCRIPTION REGULATOR-INHIBITOR complex Function and homology information
Function and homology information Regulation of gene expression in late stage (branching morphogenesis) pancreatic bud precursor cells / RUNX3 regulates NOTCH signaling / NOTCH4 Intracellular Domain Regulates Transcription / Regulation of FOXO transcriptional activity by acetylation / Regulation of gene expression by Hypoxia-inducible Factor / Nuclear events mediated by NFE2L2 / Regulation of NFE2L2 gene expression / negative regulation of transcription by RNA polymerase I / NOTCH3 Intracellular Domain Regulates Transcription / NFE2L2 regulating anti-oxidant/detoxification enzymes / TRAF6 mediated IRF7 activation / peptide-lysine-N-acetyltransferase activity / FOXO-mediated transcription of cell death genes / NFE2L2 regulating tumorigenic genes / embryonic digit morphogenesis / homeostatic process / Notch-HLH transcription pathway /
Regulation of gene expression in late stage (branching morphogenesis) pancreatic bud precursor cells / RUNX3 regulates NOTCH signaling / NOTCH4 Intracellular Domain Regulates Transcription / Regulation of FOXO transcriptional activity by acetylation / Regulation of gene expression by Hypoxia-inducible Factor / Nuclear events mediated by NFE2L2 / Regulation of NFE2L2 gene expression / negative regulation of transcription by RNA polymerase I / NOTCH3 Intracellular Domain Regulates Transcription / NFE2L2 regulating anti-oxidant/detoxification enzymes / TRAF6 mediated IRF7 activation / peptide-lysine-N-acetyltransferase activity / FOXO-mediated transcription of cell death genes / NFE2L2 regulating tumorigenic genes / embryonic digit morphogenesis / homeostatic process / Notch-HLH transcription pathway /  protein acetylation / Formation of paraxial mesoderm / positive regulation of transforming growth factor beta receptor signaling pathway / non-canonical NF-kappaB signal transduction / Zygotic genome activation (ZGA) / stimulatory C-type lectin receptor signaling pathway /
protein acetylation / Formation of paraxial mesoderm / positive regulation of transforming growth factor beta receptor signaling pathway / non-canonical NF-kappaB signal transduction / Zygotic genome activation (ZGA) / stimulatory C-type lectin receptor signaling pathway /  acetyltransferase activity / cellular response to nutrient levels / TP53 Regulates Transcription of Genes Involved in Cytochrome C Release /
acetyltransferase activity / cellular response to nutrient levels / TP53 Regulates Transcription of Genes Involved in Cytochrome C Release /  histone acetyltransferase complex / Attenuation phase / regulation of cellular response to heat /
histone acetyltransferase complex / Attenuation phase / regulation of cellular response to heat /  histone acetyltransferase activity /
histone acetyltransferase activity /  histone acetyltransferase /
histone acetyltransferase /  Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / RORA activates gene expression / NPAS4 regulates expression of target genes / Regulation of lipid metabolism by PPARalpha / CD209 (DC-SIGN) signaling / BMAL1:CLOCK,NPAS2 activates circadian gene expression / SUMOylation of transcription cofactors / Activation of gene expression by SREBF (SREBP) / Formation of the beta-catenin:TCF transactivating complex / protein destabilization / Heme signaling / Transcriptional activation of mitochondrial biogenesis /
Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / RORA activates gene expression / NPAS4 regulates expression of target genes / Regulation of lipid metabolism by PPARalpha / CD209 (DC-SIGN) signaling / BMAL1:CLOCK,NPAS2 activates circadian gene expression / SUMOylation of transcription cofactors / Activation of gene expression by SREBF (SREBP) / Formation of the beta-catenin:TCF transactivating complex / protein destabilization / Heme signaling / Transcriptional activation of mitochondrial biogenesis /  transcription coactivator binding / PPARA activates gene expression / Cytoprotection by HMOX1 / NOTCH1 Intracellular Domain Regulates Transcription / chromatin DNA binding / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants / Pre-NOTCH Transcription and Translation / Transcriptional regulation of white adipocyte differentiation / Activation of anterior HOX genes in hindbrain development during early embryogenesis / positive regulation of protein localization to nucleus / transcription corepressor activity / cellular response to UV / rhythmic process /
transcription coactivator binding / PPARA activates gene expression / Cytoprotection by HMOX1 / NOTCH1 Intracellular Domain Regulates Transcription / chromatin DNA binding / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants / Pre-NOTCH Transcription and Translation / Transcriptional regulation of white adipocyte differentiation / Activation of anterior HOX genes in hindbrain development during early embryogenesis / positive regulation of protein localization to nucleus / transcription corepressor activity / cellular response to UV / rhythmic process /  Circadian Clock /
Circadian Clock /  p53 binding / TRAF3-dependent IRF activation pathway / HATs acetylate histones / protein-containing complex assembly / DNA-binding transcription factor binding / Estrogen-dependent gene expression /
p53 binding / TRAF3-dependent IRF activation pathway / HATs acetylate histones / protein-containing complex assembly / DNA-binding transcription factor binding / Estrogen-dependent gene expression /  transcription regulator complex / RNA polymerase II-specific DNA-binding transcription factor binding / damaged DNA binding /
transcription regulator complex / RNA polymerase II-specific DNA-binding transcription factor binding / damaged DNA binding /  transcription coactivator activity / response to hypoxia /
transcription coactivator activity / response to hypoxia /  nuclear body /
nuclear body /  chromatin binding /
chromatin binding /  chromatin / regulation of DNA-templated transcription / SARS-CoV-2 activates/modulates innate and adaptive immune responses / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II /
chromatin / regulation of DNA-templated transcription / SARS-CoV-2 activates/modulates innate and adaptive immune responses / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II /  signal transduction / positive regulation of transcription by RNA polymerase II / zinc ion binding /
signal transduction / positive regulation of transcription by RNA polymerase II / zinc ion binding /  nucleoplasm /
nucleoplasm /  nucleus /
nucleus /  cytosol /
cytosol /  cytoplasm
cytoplasm
 Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.41 Å
molecular replacement / Resolution: 1.41 Å  Authors
Authors Citation
Citation Journal: J. Med. Chem. / Year: 2017
Journal: J. Med. Chem. / Year: 2017 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5w0e.cif.gz
5w0e.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5w0e.ent.gz
pdb5w0e.ent.gz PDB format
PDB format 5w0e.json.gz
5w0e.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/w0/5w0e
https://data.pdbj.org/pub/pdb/validation_reports/w0/5w0e ftp://data.pdbj.org/pub/pdb/validation_reports/w0/5w0e
ftp://data.pdbj.org/pub/pdb/validation_reports/w0/5w0e



 Links
Links Assembly
Assembly
 Components
Components

 Homo sapiens (human) / Gene: CREBBP, CBP / Production host:
Homo sapiens (human) / Gene: CREBBP, CBP / Production host: 
 Escherichia coli (E. coli) / References: UniProt: Q92793,
Escherichia coli (E. coli) / References: UniProt: Q92793,  histone acetyltransferase
histone acetyltransferase Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  SSRF
SSRF  / Beamline: BL17B1 / Wavelength: 0.979 Å
/ Beamline: BL17B1 / Wavelength: 0.979 Å : 0.979 Å / Relative weight: 1
: 0.979 Å / Relative weight: 1 
 molecular replacement
molecular replacement Processing
Processing :
:  MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller









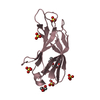


 PDBj
PDBj

















