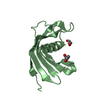
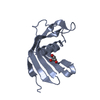
Entry Database : PDB / ID : 5o46Title Crystal structure of Iristatin, a secreted salivary cystatin from the hard tick Ixodes ricinus Iristatin Keywords / / / / Function / homology / / / / / / / / Biological species Ixodes ricinus (castor bean tick)Method / / / Resolution : 1.76 Å Authors Busa, M. / Rezacova, P. / Kotal, J. / Kotsyfakis, M. / Mares, M. Funding support Organization Grant number Country Czech Science Foundation 13-11043S Ministry of Education, Youth and Sports of the Czech Republic InterBioMed LO1302 INSTITUTE OF ORGANIC CHEMISTRY AND BIOCHEMISTRY AS CR V.V.I. RVO 61388963 Institute of Parasitology, the Czech Academy of Sciences RVO 60077344 Czech Science Foundation P502/12/2409 Grant Agency of the University of South Bohemia in Ceske Budejovice 038/2016/P European Union Marie Curie Reintegration grant PIRG07-GA-2010-268177
Journal : Cell.Mol.Life Sci. / Year : 2019Title : The structure and function of Iristatin, a novel immunosuppressive tick salivary cystatin.Authors : Kotal, J. / Stergiou, N. / Busa, M. / Chlastakova, A. / Berankova, Z. / Rezacova, P. / Langhansova, H. / Schwarz, A. / Calvo, E. / Kopecky, J. / Mares, M. / Schmitt, E. / Chmelar, J. / Kotsyfakis, M. History Deposition May 26, 2017 Deposition site / Processing site Revision 1.0 Jun 13, 2018 Provider / Type Revision 1.1 Jun 26, 2019 Group / Database references / Category / citation_author / pdbx_database_procItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year Revision 1.2 Jan 17, 2024 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords cystatin /
cystatin /  protease inhibitor /
protease inhibitor /  tick /
tick /  saliva
saliva Cystatin domain / Cystatin-like domain /
Cystatin domain / Cystatin-like domain /  Cystatin domain / Nuclear Transport Factor 2; Chain: A, - #10 / cysteine-type endopeptidase inhibitor activity / Nuclear Transport Factor 2; Chain: A, / Roll / Alpha Beta / Iristatin
Cystatin domain / Nuclear Transport Factor 2; Chain: A, - #10 / cysteine-type endopeptidase inhibitor activity / Nuclear Transport Factor 2; Chain: A, / Roll / Alpha Beta / Iristatin Function and homology information
Function and homology information
 Ixodes ricinus (castor bean tick)
Ixodes ricinus (castor bean tick) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.76 Å
MOLECULAR REPLACEMENT / Resolution: 1.76 Å  Authors
Authors Czech Republic, 7items
Czech Republic, 7items  Citation
Citation Journal: Cell.Mol.Life Sci. / Year: 2019
Journal: Cell.Mol.Life Sci. / Year: 2019 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5o46.cif.gz
5o46.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5o46.ent.gz
pdb5o46.ent.gz PDB format
PDB format 5o46.json.gz
5o46.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/o4/5o46
https://data.pdbj.org/pub/pdb/validation_reports/o4/5o46 ftp://data.pdbj.org/pub/pdb/validation_reports/o4/5o46
ftp://data.pdbj.org/pub/pdb/validation_reports/o4/5o46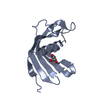
 Links
Links Assembly
Assembly


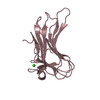
 Components
Components
 Ixodes ricinus (castor bean tick) / Production host:
Ixodes ricinus (castor bean tick) / Production host: 
 Escherichia coli (E. coli) / References: UniProt: A0A3B6UEB6*PLUS
Escherichia coli (E. coli) / References: UniProt: A0A3B6UEB6*PLUS Glycerol
Glycerol Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  BESSY
BESSY  / Beamline: 14.1 / Wavelength: 0.9184 Å
/ Beamline: 14.1 / Wavelength: 0.9184 Å : 0.9184 Å / Relative weight: 1
: 0.9184 Å / Relative weight: 1  Processing
Processing :
:  MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller









 PDBj
PDBj


