+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5mox | ||||||
|---|---|---|---|---|---|---|---|
| Title | OXA-10 Avibactam complex with bound CO2 | ||||||
 Components Components | Beta-lactamase OXA-10 | ||||||
 Keywords Keywords |  HYDROLASE / HYDROLASE /  antibiotic resistance antibiotic resistance | ||||||
| Function / homology |  Function and homology information Function and homology information penicillin binding / antibiotic catabolic process / penicillin binding / antibiotic catabolic process /  beta-lactamase activity / beta-lactamase activity /  beta-lactamase / response to antibiotic beta-lactamase / response to antibioticSimilarity search - Function | ||||||
| Biological species |   Pseudomonas aeruginosa (bacteria) Pseudomonas aeruginosa (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.41 Å molecular replacement / Resolution: 1.41 Å | ||||||
 Authors Authors | Brem, J. | ||||||
 Citation Citation |  Journal: Org. Biomol. Chem. / Year: 2017 Journal: Org. Biomol. Chem. / Year: 2017Title: (13)C-Carbamylation as a mechanistic probe for the inhibition of class D beta-lactamases by avibactam and halide ions. Authors: Lohans, C.T. / Wang, D.Y. / Jorgensen, C. / Cahill, S.T. / Clifton, I.J. / McDonough, M.A. / Oswin, H.P. / Spencer, J. / Domene, C. / Claridge, T.D.W. / Brem, J. / Schofield, C.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5mox.cif.gz 5mox.cif.gz | 220.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5mox.ent.gz pdb5mox.ent.gz | 174.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5mox.json.gz 5mox.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/mo/5mox https://data.pdbj.org/pub/pdb/validation_reports/mo/5mox ftp://data.pdbj.org/pub/pdb/validation_reports/mo/5mox ftp://data.pdbj.org/pub/pdb/validation_reports/mo/5mox | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5mmyC  5mnuC  5mozC  5fq9S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 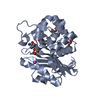
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 27655.488 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pseudomonas aeruginosa (bacteria) / Gene: bla, oxa10, pse2 / Production host: Pseudomonas aeruginosa (bacteria) / Gene: bla, oxa10, pse2 / Production host:   Escherichia coli (E. coli) / References: UniProt: P14489, Escherichia coli (E. coli) / References: UniProt: P14489,  beta-lactamase beta-lactamase |
|---|
-Non-polymers , 5 types, 619 molecules 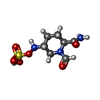







| #2: Chemical |  Avibactam Avibactam#3: Chemical | #4: Chemical | ChemComp-CO2 / |  Carbon dioxide Carbon dioxide#5: Chemical |  Glycerol Glycerol#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.2 Å3/Da / Density % sol: 61 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 0.2M Sodium citrate tribasic dihydrate, 0.1M Bis-Tris propane 7.5, 20 % w/v PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.9795 Å / Beamline: I04 / Wavelength: 0.9795 Å |
| Detector | Type: DECTRIS PILATUS3 S 6M / Detector: PIXEL / Date: Jun 26, 2016 / Details: mirror |
| Radiation | Monochromator: double crystal / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9795 Å / Relative weight: 1 : 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 1.41→76.76 Å / Num. obs: 115816 / % possible obs: 100 % / Observed criterion σ(I): 1.2 / Redundancy: 12.9 % / Rmerge(I) obs: 0.179 / Net I/σ(I): 9.5 |
| Reflection shell | Resolution: 1.41→1.43 Å / Redundancy: 12.6 % / Rmerge(I) obs: 3.083 / Mean I/σ(I) obs: 1.2 / Num. unique all: 5742 / CC1/2: 0.511 / % possible all: 100 |
-Phasing
Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5FQ9 Resolution: 1.41→76.76 Å / Cross valid method: FREE R-VALUE
| ||||||||||||||||
| Displacement parameters | Biso max: 90.63 Å2 / Biso mean: 19.0961 Å2 / Biso min: 5.54 Å2 | ||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.41→76.76 Å
|
 Movie
Movie Controller
Controller












 PDBj
PDBj




