+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ks2 | ||||||
|---|---|---|---|---|---|---|---|
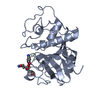
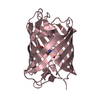
| Title | RAWV_CTD (Helix form) of 16S/23S 2'-O-methyltransferase TlyA | ||||||
 Components Components | 16S/23S rRNA (cytidine-2'-O)-methyltransferase TlyA | ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  Methyltransferase / Methyltransferase /  hemolysin / hemolysin /  ribosome / ribosome /  antibiotic sensitivity antibiotic sensitivity | ||||||
| Function / homology |  Function and homology information Function and homology information 16S rRNA (cytidine1409-2'-O)-methyltransferase / 16S rRNA (cytidine1409-2'-O)-methyltransferase /  23S rRNA (cytidine1920-2'-O)-methyltransferase / hemolysis by symbiont of host erythrocytes / rRNA methyltransferase activity / rRNA methylation / 23S rRNA (cytidine1920-2'-O)-methyltransferase / hemolysis by symbiont of host erythrocytes / rRNA methyltransferase activity / rRNA methylation /  toxin activity / killing of cells of another organism / host cell plasma membrane / toxin activity / killing of cells of another organism / host cell plasma membrane /  RNA binding / extracellular region ... RNA binding / extracellular region ... 16S rRNA (cytidine1409-2'-O)-methyltransferase / 16S rRNA (cytidine1409-2'-O)-methyltransferase /  23S rRNA (cytidine1920-2'-O)-methyltransferase / hemolysis by symbiont of host erythrocytes / rRNA methyltransferase activity / rRNA methylation / 23S rRNA (cytidine1920-2'-O)-methyltransferase / hemolysis by symbiont of host erythrocytes / rRNA methyltransferase activity / rRNA methylation /  toxin activity / killing of cells of another organism / host cell plasma membrane / toxin activity / killing of cells of another organism / host cell plasma membrane /  RNA binding / extracellular region / RNA binding / extracellular region /  membrane / membrane /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Mycobacterium tuberculosis (bacteria) Mycobacterium tuberculosis (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.18 Å MOLECULAR REPLACEMENT / Resolution: 2.18 Å | ||||||
 Authors Authors | Kuiper, E.G. / Conn, G.L. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: J. Biol. Chem. / Year: 2017 Journal: J. Biol. Chem. / Year: 2017Title: A Novel Motif for S-Adenosyl-l-methionine Binding by the Ribosomal RNA Methyltransferase TlyA from Mycobacterium tuberculosis. Authors: Witek, M.A. / Kuiper, E.G. / Minten, E. / Crispell, E.K. / Conn, G.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ks2.cif.gz 5ks2.cif.gz | 93.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ks2.ent.gz pdb5ks2.ent.gz | 69.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ks2.json.gz 5ks2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ks/5ks2 https://data.pdbj.org/pub/pdb/validation_reports/ks/5ks2 ftp://data.pdbj.org/pub/pdb/validation_reports/ks/5ks2 ftp://data.pdbj.org/pub/pdb/validation_reports/ks/5ks2 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5eovSC  5kygC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Details | Monomer as determined by gel filtration |
- Components
Components
| #1: Protein | Mass: 23690.039 Da / Num. of mol.: 1 / Fragment: residues 60-268 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mycobacterium tuberculosis (bacteria) / Strain: ATCC 25618 / H37Rv / Gene: tlyA, Rv1694 / Production host: Mycobacterium tuberculosis (bacteria) / Strain: ATCC 25618 / H37Rv / Gene: tlyA, Rv1694 / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: P9WJ63,  23S rRNA (cytidine1920-2'-O)-methyltransferase, 23S rRNA (cytidine1920-2'-O)-methyltransferase,  16S rRNA (cytidine1409-2'-O)-methyltransferase 16S rRNA (cytidine1409-2'-O)-methyltransferase |
|---|---|
| #2: Chemical | ChemComp-CL /  Chloride Chloride |
| #3: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.82 Å3/Da / Density % sol: 36.28 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 7.5 / Details: 0.3 M Hepes pH 7.5 2.8M NaCl |
-Data collection
| Diffraction | Mean temperature: 80 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-ID / Wavelength: 1 Å / Beamline: 22-ID / Wavelength: 1 Å |
| Detector | Type: RAYONIX MX300-HS / Detector: CCD / Date: Feb 21, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.18→51.6 Å / Num. obs: 10172 / % possible obs: 99.9 % / Redundancy: 13.2 % / CC1/2: 0.558 / Rmerge(I) obs: 0.319 / Net I/σ(I): 19.2 |
| Reflection shell | Resolution: 2.18→2.24 Å / Redundancy: 5.6 % / Rmerge(I) obs: 0.984 / Mean I/σ(I) obs: 4.7 / CC1/2: 0.588 / % possible all: 99.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5EOV Resolution: 2.18→51.6 Å / Cor.coef. Fo:Fc: 0.957 / Cor.coef. Fo:Fc free: 0.923 / SU B: 12.378 / SU ML: 0.157 / Cross valid method: FREE R-VALUE / σ(F): 0 / ESU R: 0.322 / ESU R Free: 0.23 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.3 Å | ||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 106.72 Å2 / Biso mean: 34.6 Å2 / Biso min: 18.54 Å2
| ||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.18→51.6 Å
| ||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.18→2.237 Å / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 17.026 Å / Origin y: -11.176 Å / Origin z: 3.125 Å
| ||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: chain A |
 Movie
Movie Controller
Controller











 PDBj
PDBj





