+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5jer | ||||||
|---|---|---|---|---|---|---|---|
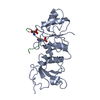
| Title | Structure of Rotavirus NSP1 bound to IRF-3 | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  IMMUNE SYSTEM / Viral Immunity IMMUNE SYSTEM / Viral Immunity | ||||||
| Function / homology |  Function and homology information Function and homology informationIRF3 mediated activation of type 1 IFN / MDA-5 signaling pathway / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF7 activity / macrophage apoptotic process / host cytoskeleton / programmed necrotic cell death / TRIF-dependent toll-like receptor signaling pathway / LRR FLII-interacting protein 1 (LRRFIP1) activates type I IFN production / IRF3-mediated induction of type I IFN / positive regulation of type I interferon-mediated signaling pathway ...IRF3 mediated activation of type 1 IFN / MDA-5 signaling pathway / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF7 activity / macrophage apoptotic process / host cytoskeleton / programmed necrotic cell death / TRIF-dependent toll-like receptor signaling pathway / LRR FLII-interacting protein 1 (LRRFIP1) activates type I IFN production / IRF3-mediated induction of type I IFN / positive regulation of type I interferon-mediated signaling pathway /  mRNA transcription / toll-like receptor 4 signaling pathway / TRAF6 mediated IRF7 activation / DNA-binding transcription activator activity / mRNA transcription / toll-like receptor 4 signaling pathway / TRAF6 mediated IRF7 activation / DNA-binding transcription activator activity /  immune system process / type I interferon-mediated signaling pathway / cellular response to exogenous dsRNA / cytoplasmic pattern recognition receptor signaling pathway / positive regulation of interferon-alpha production / antiviral innate immune response / positive regulation of type I interferon production / lipopolysaccharide-mediated signaling pathway / TICAM1-dependent activation of IRF3/IRF7 / Regulation of innate immune responses to cytosolic DNA / positive regulation of interferon-beta production / Activation of IRF3, IRF7 mediated by TBK1, IKKε (IKBKE) / Negative regulators of DDX58/IFIH1 signaling / promoter-specific chromatin binding / DDX58/IFIH1-mediated induction of interferon-alpha/beta / ISG15 antiviral mechanism / cellular response to virus / DNA-binding transcription repressor activity, RNA polymerase II-specific / SARS-CoV-1 activates/modulates innate immune responses / Interferon gamma signaling / Interferon alpha/beta signaling / sequence-specific double-stranded DNA binding / TRAF3-dependent IRF activation pathway / immune system process / type I interferon-mediated signaling pathway / cellular response to exogenous dsRNA / cytoplasmic pattern recognition receptor signaling pathway / positive regulation of interferon-alpha production / antiviral innate immune response / positive regulation of type I interferon production / lipopolysaccharide-mediated signaling pathway / TICAM1-dependent activation of IRF3/IRF7 / Regulation of innate immune responses to cytosolic DNA / positive regulation of interferon-beta production / Activation of IRF3, IRF7 mediated by TBK1, IKKε (IKBKE) / Negative regulators of DDX58/IFIH1 signaling / promoter-specific chromatin binding / DDX58/IFIH1-mediated induction of interferon-alpha/beta / ISG15 antiviral mechanism / cellular response to virus / DNA-binding transcription repressor activity, RNA polymerase II-specific / SARS-CoV-1 activates/modulates innate immune responses / Interferon gamma signaling / Interferon alpha/beta signaling / sequence-specific double-stranded DNA binding / TRAF3-dependent IRF activation pathway /  regulation of inflammatory response / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF3 activity / DNA-binding transcription activator activity, RNA polymerase II-specific / regulation of apoptotic process / defense response to virus / positive regulation of canonical NF-kappaB signal transduction / host cell cytoplasm / sequence-specific DNA binding / DNA-binding transcription factor activity, RNA polymerase II-specific / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / protein domain specific binding / apoptotic process / DNA damage response / regulation of inflammatory response / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of IRF3 activity / DNA-binding transcription activator activity, RNA polymerase II-specific / regulation of apoptotic process / defense response to virus / positive regulation of canonical NF-kappaB signal transduction / host cell cytoplasm / sequence-specific DNA binding / DNA-binding transcription factor activity, RNA polymerase II-specific / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / protein domain specific binding / apoptotic process / DNA damage response /  chromatin / regulation of transcription by RNA polymerase II / SARS-CoV-2 activates/modulates innate and adaptive immune responses / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / chromatin / regulation of transcription by RNA polymerase II / SARS-CoV-2 activates/modulates innate and adaptive immune responses / protein homodimerization activity / positive regulation of transcription by RNA polymerase II /  mitochondrion / mitochondrion /  DNA binding / DNA binding /  RNA binding / RNA binding /  nucleoplasm / identical protein binding / nucleoplasm / identical protein binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Rotavirus A Rotavirus A  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 2.913 Å SYNCHROTRON / Resolution: 2.913 Å | ||||||
 Authors Authors | Zhao, B. / Li, P. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2016 Journal: Proc.Natl.Acad.Sci.USA / Year: 2016Title: Structural basis for concerted recruitment and activation of IRF-3 by innate immune adaptor proteins. Authors: Zhao, B. / Shu, C. / Gao, X. / Sankaran, B. / Du, F. / Shelton, C.L. / Herr, A.B. / Ji, J.Y. / Li, P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5jer.cif.gz 5jer.cif.gz | 391.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5jer.ent.gz pdb5jer.ent.gz | 327.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5jer.json.gz 5jer.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/je/5jer https://data.pdbj.org/pub/pdb/validation_reports/je/5jer ftp://data.pdbj.org/pub/pdb/validation_reports/je/5jer ftp://data.pdbj.org/pub/pdb/validation_reports/je/5jer | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein/peptide | Mass: 2257.318 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rotavirus A / Production host: Rotavirus A / Production host:   Escherichia coli (E. coli) / References: UniProt: Q99FX5*PLUS Escherichia coli (E. coli) / References: UniProt: Q99FX5*PLUS#2: Protein |  IRF3 / IRF-3 IRF3 / IRF-3Mass: 26901.416 Da / Num. of mol.: 4 / Fragment: UNP residues 189-427 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: IRF3 / Production host: Homo sapiens (human) / Gene: IRF3 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q14653 Escherichia coli (E. coli) / References: UniProt: Q14653 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.13 Å3/Da / Density % sol: 42.16 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / Details: 0.1 M Bis-Tris pH 5.5, 200 mM MgCl2, 23% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 120 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.1 / Wavelength: 1 Å / Beamline: 5.0.1 / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Jul 3, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.9→47.94 Å / Num. obs: 22409 / % possible obs: 100 % / Redundancy: 7.1 % / Net I/σ(I): 15.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.913→47.94 Å / SU ML: 0.37 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 25.1
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.913→47.94 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj





