+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ix0 | ||||||
|---|---|---|---|---|---|---|---|
| Title | HDAC2 WITH LIGAND BRD7232 | ||||||
 Components Components | Histone deacetylase 2 | ||||||
 Keywords Keywords |  HYDROLASE / HDAC HISTONE DEACETYLASE HYDROLASE / HDAC HISTONE DEACETYLASE | ||||||
| Function / homology |  Function and homology information Function and homology informationprotein de-2-hydroxyisobutyrylase activity / positive regulation of male mating behavior / negative regulation of peptidyl-lysine acetylation / p75NTR negatively regulates cell cycle via SC1 / epidermal cell differentiation / negative regulation of dendritic spine development / histone decrotonylase activity / fungiform papilla formation / behavioral response to ethanol / negative regulation of MHC class II biosynthetic process ...protein de-2-hydroxyisobutyrylase activity / positive regulation of male mating behavior / negative regulation of peptidyl-lysine acetylation / p75NTR negatively regulates cell cycle via SC1 / epidermal cell differentiation / negative regulation of dendritic spine development / histone decrotonylase activity / fungiform papilla formation / behavioral response to ethanol / negative regulation of MHC class II biosynthetic process / NuRD complex / positive regulation of interleukin-1 production / regulation of cell fate specification / negative regulation of stem cell population maintenance / EGR2 and SOX10-mediated initiation of Schwann cell myelination / negative regulation of transcription by competitive promoter binding / ESC/E(Z) complex /  regulation of stem cell differentiation / STAT3 nuclear events downstream of ALK signaling / cellular response to dopamine / regulation of stem cell differentiation / STAT3 nuclear events downstream of ALK signaling / cellular response to dopamine /  histone deacetylase / cardiac muscle hypertrophy / protein lysine deacetylase activity / positive regulation of signaling receptor activity / response to caffeine / histone deacetylase / cardiac muscle hypertrophy / protein lysine deacetylase activity / positive regulation of signaling receptor activity / response to caffeine /  Hydrolases; Acting on carbon-nitrogen bonds, other than peptide bonds; In linear amides / Hydrolases; Acting on carbon-nitrogen bonds, other than peptide bonds; In linear amides /  histone deacetylase activity / embryonic digit morphogenesis / positive regulation of oligodendrocyte differentiation / positive regulation of stem cell population maintenance / Sin3-type complex / Notch-HLH transcription pathway / dendrite development / eyelid development in camera-type eye / odontogenesis of dentin-containing tooth / positive regulation of proteolysis / RNA Polymerase I Transcription Initiation / response to amyloid-beta / histone deacetylase activity / embryonic digit morphogenesis / positive regulation of oligodendrocyte differentiation / positive regulation of stem cell population maintenance / Sin3-type complex / Notch-HLH transcription pathway / dendrite development / eyelid development in camera-type eye / odontogenesis of dentin-containing tooth / positive regulation of proteolysis / RNA Polymerase I Transcription Initiation / response to amyloid-beta /  histone deacetylase complex / hair follicle placode formation / Regulation of MECP2 expression and activity / histone deacetylase complex / hair follicle placode formation / Regulation of MECP2 expression and activity /  NF-kappaB binding / positive regulation of collagen biosynthetic process / response to hyperoxia / positive regulation of epithelial to mesenchymal transition / FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes / heterochromatin formation / MECP2 regulates neuronal receptors and channels / cellular response to retinoic acid / positive regulation of tyrosine phosphorylation of STAT protein / Regulation of TP53 Activity through Acetylation / cellular response to transforming growth factor beta stimulus / response to amphetamine / NF-kappaB binding / positive regulation of collagen biosynthetic process / response to hyperoxia / positive regulation of epithelial to mesenchymal transition / FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes / heterochromatin formation / MECP2 regulates neuronal receptors and channels / cellular response to retinoic acid / positive regulation of tyrosine phosphorylation of STAT protein / Regulation of TP53 Activity through Acetylation / cellular response to transforming growth factor beta stimulus / response to amphetamine /  heat shock protein binding / SUMOylation of chromatin organization proteins / negative regulation of cell migration / response to cocaine / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / response to nicotine / Regulation of PTEN gene transcription / promoter-specific chromatin binding / HDACs deacetylate histones / negative regulation of transforming growth factor beta receptor signaling pathway / circadian regulation of gene expression / protein modification process / NoRC negatively regulates rRNA expression / negative regulation of DNA-binding transcription factor activity / NOTCH1 Intracellular Domain Regulates Transcription / cellular response to hydrogen peroxide / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants / heat shock protein binding / SUMOylation of chromatin organization proteins / negative regulation of cell migration / response to cocaine / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / response to nicotine / Regulation of PTEN gene transcription / promoter-specific chromatin binding / HDACs deacetylate histones / negative regulation of transforming growth factor beta receptor signaling pathway / circadian regulation of gene expression / protein modification process / NoRC negatively regulates rRNA expression / negative regulation of DNA-binding transcription factor activity / NOTCH1 Intracellular Domain Regulates Transcription / cellular response to hydrogen peroxide / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants /  histone deacetylase binding / positive regulation of tumor necrosis factor production / negative regulation of neuron projection development / cellular response to heat / Factors involved in megakaryocyte development and platelet production / histone deacetylase binding / positive regulation of tumor necrosis factor production / negative regulation of neuron projection development / cellular response to heat / Factors involved in megakaryocyte development and platelet production /  histone binding / RNA polymerase II-specific DNA-binding transcription factor binding / Potential therapeutics for SARS / response to lipopolysaccharide / histone binding / RNA polymerase II-specific DNA-binding transcription factor binding / Potential therapeutics for SARS / response to lipopolysaccharide /  chromosome, telomeric region / chromosome, telomeric region /  chromatin remodeling / response to xenobiotic stimulus / negative regulation of DNA-templated transcription / chromatin remodeling / response to xenobiotic stimulus / negative regulation of DNA-templated transcription /  chromatin binding / positive regulation of cell population proliferation / chromatin binding / positive regulation of cell population proliferation /  chromatin / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II / chromatin / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / negative regulation of transcription by RNA polymerase II /  enzyme binding / positive regulation of transcription by RNA polymerase II / protein-containing complex / enzyme binding / positive regulation of transcription by RNA polymerase II / protein-containing complex /  RNA binding / RNA binding /  nucleoplasm / nucleoplasm /  nucleus / nucleus /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.72 Å MOLECULAR REPLACEMENT / Resolution: 1.72 Å | ||||||
 Authors Authors | Steinbacher, S. | ||||||
 Citation Citation |  Journal: Bioorg.Med.Chem. / Year: 2016 Journal: Bioorg.Med.Chem. / Year: 2016Title: Kinetic and structural insights into the binding of histone deacetylase 1 and 2 (HDAC1, 2) inhibitors. Authors: Wagner, F.F. / Weiwer, M. / Steinbacher, S. / Schomburg, A. / Reinemer, P. / Gale, J.P. / Campbell, A.J. / Fisher, S.L. / Zhao, W.N. / Reis, S.A. / Hennig, K.M. / Thomas, M. / Muller, P. / ...Authors: Wagner, F.F. / Weiwer, M. / Steinbacher, S. / Schomburg, A. / Reinemer, P. / Gale, J.P. / Campbell, A.J. / Fisher, S.L. / Zhao, W.N. / Reis, S.A. / Hennig, K.M. / Thomas, M. / Muller, P. / Jefson, M.R. / Fass, D.M. / Haggarty, S.J. / Zhang, Y.L. / Holson, E.B. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ix0.cif.gz 5ix0.cif.gz | 473.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ix0.ent.gz pdb5ix0.ent.gz | 387.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ix0.json.gz 5ix0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ix/5ix0 https://data.pdbj.org/pub/pdb/validation_reports/ix/5ix0 ftp://data.pdbj.org/pub/pdb/validation_reports/ix/5ix0 ftp://data.pdbj.org/pub/pdb/validation_reports/ix/5ix0 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 |
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein |  / HD2 / HD2Mass: 42286.141 Da / Num. of mol.: 3 / Fragment: UNP residues 7-375 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: HDAC2 / Production host: Homo sapiens (human) / Gene: HDAC2 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q92769, Escherichia coli (E. coli) / References: UniProt: Q92769,  histone deacetylase histone deacetylase |
|---|
-Non-polymers , 9 types, 872 molecules 

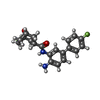


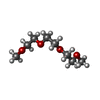











| #2: Chemical | | #3: Chemical | ChemComp-CA / #4: Chemical | #5: Chemical | ChemComp-PEG /  Diethylene glycol Diethylene glycol#6: Chemical |  Polyethylene glycol Polyethylene glycol#7: Chemical |  Triethylene glycol dimethyl ether Triethylene glycol dimethyl ether#8: Chemical | ChemComp-PGE /  Polyethylene glycol Polyethylene glycol#9: Chemical |  Ethylene glycol Ethylene glycol#10: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.51 Å3/Da / Density % sol: 51.07 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 5.5 / Details: PEG 400, potassium phosphate |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 1 Å / Beamline: X06SA / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Nov 18, 2013 / Details: Dynamically bendable mirror |
| Radiation | Monochromator: LN2 cooled fixed-exit Si(111) monochromator / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.72→79.85 Å / Num. obs: 133389 / % possible obs: 100 % / Observed criterion σ(I): 0 / Redundancy: 8.2 % / Rmerge(I) obs: 0.08 / Net I/σ(I): 2.81 |
| Reflection shell | Resolution: 1.72→1.76 Å / Redundancy: 8.4 % / Rmerge(I) obs: 0.934 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 1.72→48.736 Å / Cor.coef. Fo:Fc: 0.962 / Cor.coef. Fo:Fc free: 0.949 / SU B: 4.784 / SU ML: 0.076 / Cross valid method: THROUGHOUT / ESU R: 0.102 / ESU R Free: 0.095 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS MOLECULAR REPLACEMENT / Resolution: 1.72→48.736 Å / Cor.coef. Fo:Fc: 0.962 / Cor.coef. Fo:Fc free: 0.949 / SU B: 4.784 / SU ML: 0.076 / Cross valid method: THROUGHOUT / ESU R: 0.102 / ESU R Free: 0.095 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 27.193 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.72→48.736 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj
































