[English] 日本語
 Yorodumi
Yorodumi- PDB-5hnk: Crystal structure of T5Fen in complex intact substrate and metal ions. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5hnk | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of T5Fen in complex intact substrate and metal ions. | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  HYDROLASE / enzyme-DNA complex / HYDROLASE / enzyme-DNA complex /  flap endonuclease / flap endonuclease /  metalloenzyme. metalloenzyme. | ||||||
| Function / homology |  Function and homology information Function and homology information viral replication complex / viral replication complex /  exodeoxyribonuclease (lambda-induced) / late viral transcription / exodeoxyribonuclease (lambda-induced) / late viral transcription /  DNA replication, Okazaki fragment processing / double-stranded DNA 5'-3' DNA exonuclease activity / DNA exonuclease activity / double-stranded DNA endonuclease activity / 5'-flap endonuclease activity / 5'-3' exonuclease activity / viral DNA genome replication ... DNA replication, Okazaki fragment processing / double-stranded DNA 5'-3' DNA exonuclease activity / DNA exonuclease activity / double-stranded DNA endonuclease activity / 5'-flap endonuclease activity / 5'-3' exonuclease activity / viral DNA genome replication ... viral replication complex / viral replication complex /  exodeoxyribonuclease (lambda-induced) / late viral transcription / exodeoxyribonuclease (lambda-induced) / late viral transcription /  DNA replication, Okazaki fragment processing / double-stranded DNA 5'-3' DNA exonuclease activity / DNA exonuclease activity / double-stranded DNA endonuclease activity / 5'-flap endonuclease activity / 5'-3' exonuclease activity / viral DNA genome replication / DNA replication, Okazaki fragment processing / double-stranded DNA 5'-3' DNA exonuclease activity / DNA exonuclease activity / double-stranded DNA endonuclease activity / 5'-flap endonuclease activity / 5'-3' exonuclease activity / viral DNA genome replication /  Hydrolases; Acting on ester bonds; Exodeoxyribonucleases producing 5'-phosphomonoesters / 5'-3' DNA exonuclease activity / Hydrolases; Acting on ester bonds; Exodeoxyribonucleases producing 5'-phosphomonoesters / 5'-3' DNA exonuclease activity /  DNA binding / DNA binding /  metal ion binding metal ion bindingSimilarity search - Function | ||||||
| Biological species |   Escherichia phage T5 (virus) Escherichia phage T5 (virus)synthetic construct (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.22 Å MOLECULAR REPLACEMENT / Resolution: 2.22 Å | ||||||
 Authors Authors | Almalki, F.A. / Feng, M. / Zhang, J. / Sedelnikova, S.E. / Rafferty, J.B. / Sayers, J.R. / Artymiuk, P.J. | ||||||
 Citation Citation |  Journal: Nat.Struct.Mol.Biol. / Year: 2016 Journal: Nat.Struct.Mol.Biol. / Year: 2016Title: Direct observation of DNA threading in flap endonuclease complexes. Authors: AlMalki, F.A. / Flemming, C.S. / Zhang, J. / Feng, M. / Sedelnikova, S.E. / Ceska, T. / Rafferty, J.B. / Sayers, J.R. / Artymiuk, P.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5hnk.cif.gz 5hnk.cif.gz | 265.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5hnk.ent.gz pdb5hnk.ent.gz | 210.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5hnk.json.gz 5hnk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hn/5hnk https://data.pdbj.org/pub/pdb/validation_reports/hn/5hnk ftp://data.pdbj.org/pub/pdb/validation_reports/hn/5hnk ftp://data.pdbj.org/pub/pdb/validation_reports/hn/5hnk | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5hmlC  5hmmC  5hp4C  1exnS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 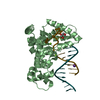
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-DNA chain / Protein , 2 types, 4 molecules XYAB
| #1: DNA chain | Mass: 3680.432 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) #2: Protein |  / 5'-exonuclease / T5FEN / D15 exonuclease / 5'-exonuclease / T5FEN / D15 exonucleaseMass: 31275.475 Da / Num. of mol.: 2 / Fragment: UNP residues 20-291 Source method: isolated from a genetically manipulated source Details: Corresponds to residues 20-291 of Uniprot P06229 but with mutation D153K Source: (gene. exp.)   Escherichia phage T5 (virus) / Gene: D15 / Plasmid: pJONEX4 Escherichia phage T5 (virus) / Gene: D15 / Plasmid: pJONEX4Details (production host): pUC19 derivative carrying a lambda promoter. Production host:   Escherichia coli (E. coli) / Strain (production host): M72 Escherichia coli (E. coli) / Strain (production host): M72References: UniProt: P06229,  exodeoxyribonuclease (lambda-induced) exodeoxyribonuclease (lambda-induced) |
|---|
-Non-polymers , 4 types, 228 molecules 






| #3: Chemical | | #4: Chemical | ChemComp-K / | #5: Chemical | ChemComp-GOL / |  Glycerol Glycerol#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.24 Å3/Da / Density % sol: 45.06 % |
|---|---|
Crystal grow | Temperature: 290 K / Method: vapor diffusion, hanging drop / pH: 5.5 Details: The concentration of both oligonucleotides was adjusted to 1.1 mM for the duplex molecule by dissolving each one in 10 mM MES pH 6.5 and 50 mM KCl. They were annealed by heating to 367K for ...Details: The concentration of both oligonucleotides was adjusted to 1.1 mM for the duplex molecule by dissolving each one in 10 mM MES pH 6.5 and 50 mM KCl. They were annealed by heating to 367K for 10 minutes and allowed to cool to room temperature. Crystals with oligonucleotide 5ov4 were grown at 290K with the D153K variant of T5Fen. The resulting T5FenD153K:5ov4 structure was determined from crystals grown in 0.2 M MgCl2, 0.1 M Bis-Tris buffer pH 5.5, 25% w/v PEG 3350. |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I24 / Wavelength: 0.977 Å / Beamline: I24 / Wavelength: 0.977 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Apr 16, 2011 Details: Please check which detector was in place at that date | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 0.977 Å / Relative weight: 1 : 0.977 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.22→44.72 Å / Num. all: 31727 / Num. obs: 31727 / % possible obs: 99.4 % / Redundancy: 5.7 % / Rpim(I) all: 0.038 / Rrim(I) all: 0.094 / Rsym value: 0.079 / Net I/av σ(I): 7.049 / Net I/σ(I): 13.4 / Num. measured all: 182387 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: 0
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1EXN Resolution: 2.22→42.19 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.932 / WRfactor Rfree: 0.2347 / WRfactor Rwork: 0.1834 / FOM work R set: 0.8335 / SU B: 14.251 / SU ML: 0.175 / SU R Cruickshank DPI: 0.3029 / SU Rfree: 0.2192 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.303 / ESU R Free: 0.219 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : WITH TLS ADDED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 154.83 Å2 / Biso mean: 48.335 Å2 / Biso min: 21.96 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.22→42.19 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.22→2.278 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj









































