[English] 日本語
 Yorodumi
Yorodumi- PDB-5g5f: Crystallographic structure of the Tau class glutathione S-transfe... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5g5f | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystallographic structure of the Tau class glutathione S-transferase MiGSTU in complex with reduced glutathione. | ||||||
 Components Components | TAU CLASS GLUTATHIONE S-TRANSFERASE | ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  GLUTATHIONE S-TRANSFERASE / TAU CLASS / MANGO / GLUTATHIONE S-TRANSFERASE / TAU CLASS / MANGO /  DETOXIFICATION / REDUCED GLUTATHIONE. DETOXIFICATION / REDUCED GLUTATHIONE. | ||||||
| Function / homology |  Function and homology information Function and homology informationtoxin catabolic process /  glutathione transferase / glutathione transferase /  glutathione transferase activity / glutathione metabolic process / glutathione transferase activity / glutathione metabolic process /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   MANGIFERA INDICA (mango) MANGIFERA INDICA (mango) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Valenzuela-Chavira, I. / Serrano-Posada, H. / Lopez-Zavala, A. / Hernandez-Paredes, J. / Sotelo-Mundo, R. | ||||||
 Citation Citation |  Journal: Biochimie / Year: 2017 Journal: Biochimie / Year: 2017Title: Insights Into Ligand Binding to a Glutathione S-Transferase from Mango: Structure, Thermodynamics and Kinetics Authors: Valenzuela-Chavira, I. / Contreras-Vergara, C.A. / Arvizu-Flores, A.A. / Serrano-Posada, H. / Lopez-Zavala, A.A. / Garcia-Orozco, K.D. / Hernandez-Paredes, J. / Rudino-Pinera, E. / ...Authors: Valenzuela-Chavira, I. / Contreras-Vergara, C.A. / Arvizu-Flores, A.A. / Serrano-Posada, H. / Lopez-Zavala, A.A. / Garcia-Orozco, K.D. / Hernandez-Paredes, J. / Rudino-Pinera, E. / Stojanoff, V. / Sotelo-Mundo, R.R. / Islas-Osuna, M.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5g5f.cif.gz 5g5f.cif.gz | 58.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5g5f.ent.gz pdb5g5f.ent.gz | 42.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5g5f.json.gz 5g5f.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/g5/5g5f https://data.pdbj.org/pub/pdb/validation_reports/g5/5g5f ftp://data.pdbj.org/pub/pdb/validation_reports/g5/5g5f ftp://data.pdbj.org/pub/pdb/validation_reports/g5/5g5f | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5g5eC  5kejC  1gwcS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 25550.686 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   MANGIFERA INDICA (mango) MANGIFERA INDICA (mango)Description: THE MIGSTU SEQUENCE WAS IDENTIFIED FROM A MANGO HADEN MESOCARP CDNA SHOTGUN-CDNA SEQUENCING PROJECT. Plasmid: PJEXPRESS404 / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3)References: UniProt: A0A1P8NWC2*PLUS,  glutathione transferase glutathione transferase | ||
|---|---|---|---|
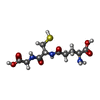
| #2: Chemical | ChemComp-GSH /  Glutathione Glutathione | ||
| #3: Chemical | ChemComp-PEG /  Diethylene glycol Diethylene glycol#4: Water | ChemComp-HOH / |  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.11 Å3/Da / Density % sol: 41.78 % / Description: NONE |
|---|---|
Crystal grow | pH: 6 Details: 0.2 M AMMONIUM ACETATE, 0.1 M BIS-TRIS PH 6.0, 25%(W/V) POLYETHYLENE GLYCOL 3350, 5 MM GSH |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source: SEALED TUBE / Type: BRUKER D8 QUEST / Wavelength: 1.54178 / Wavelength: 1.54178 Å |
| Detector | Type: BRUKER / Detector: CMOS / Date: Dec 4, 2015 / Details: HELIOS MX |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.54178 Å / Relative weight: 1 : 1.54178 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→20.3 Å / Num. obs: 9224 / % possible obs: 96.3 % / Observed criterion σ(I): 0 / Redundancy: 2 % / Biso Wilson estimate: 20.24 Å2 / Rmerge(I) obs: 0.04 / Net I/σ(I): 25 |
| Reflection shell | Resolution: 2.3→2.38 Å / Redundancy: 2 % / Rmerge(I) obs: 0.3 / Mean I/σ(I) obs: 4.3 / % possible all: 97.6 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: HOMOLOGY MODEL BASED ON THE STRUCTURE OF WHEAT TAU CLASS GLUTATHIONE S-TRANSFERASE. PDB ENTRY 1GWC. Resolution: 2.3→20.277 Å / SU ML: 0.25 / σ(F): 0 / Phase error: 22.49 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 28.7 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→20.277 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj