+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4zc4 | ||||||
|---|---|---|---|---|---|---|---|
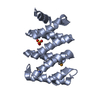
| Title | Crystal structure of LARP1-unique domain DM15 | ||||||
 Components Components | La-related protein 1 | ||||||
 Keywords Keywords |  RNA BINDING PROTEIN / RNA-binding / RNA BINDING PROTEIN / RNA-binding /  Heat-like / Heat-like /  mRNA / helical repeat mRNA / helical repeat | ||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to rapamycin / translation activator activity / eukaryotic initiation factor 4E binding /  RNA cap binding / TORC1 signaling / response to amino acid starvation / RNA 7-methylguanosine cap binding / mRNA stabilization / post-transcriptional regulation of gene expression / positive regulation of macroautophagy ...cellular response to rapamycin / translation activator activity / eukaryotic initiation factor 4E binding / RNA cap binding / TORC1 signaling / response to amino acid starvation / RNA 7-methylguanosine cap binding / mRNA stabilization / post-transcriptional regulation of gene expression / positive regulation of macroautophagy ...cellular response to rapamycin / translation activator activity / eukaryotic initiation factor 4E binding /  RNA cap binding / TORC1 signaling / response to amino acid starvation / RNA 7-methylguanosine cap binding / mRNA stabilization / post-transcriptional regulation of gene expression / positive regulation of macroautophagy / RNA cap binding / TORC1 signaling / response to amino acid starvation / RNA 7-methylguanosine cap binding / mRNA stabilization / post-transcriptional regulation of gene expression / positive regulation of macroautophagy /  ribosomal small subunit binding / ribosomal small subunit binding /  TOR signaling / positive regulation of translational initiation / positive regulation of viral genome replication / negative regulation of translational initiation / translational initiation / TOR signaling / positive regulation of translational initiation / positive regulation of viral genome replication / negative regulation of translational initiation / translational initiation /  translation initiation factor binding / mRNA 3'-UTR binding / positive regulation of translation / mRNA 5'-UTR binding / cytoplasmic stress granule / cell population proliferation / negative regulation of translation / translation initiation factor binding / mRNA 3'-UTR binding / positive regulation of translation / mRNA 5'-UTR binding / cytoplasmic stress granule / cell population proliferation / negative regulation of translation /  cadherin binding / SARS-CoV-2 activates/modulates innate and adaptive immune responses / cadherin binding / SARS-CoV-2 activates/modulates innate and adaptive immune responses /  RNA binding / RNA binding /  membrane / membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 1.86 Å MAD / Resolution: 1.86 Å | ||||||
 Authors Authors | Lahr, R.M. / Berman, A.J. | ||||||
 Citation Citation |  Journal: Nucleic Acids Res. / Year: 2015 Journal: Nucleic Acids Res. / Year: 2015Title: The La-related protein 1-specific domain repurposes HEAT-like repeats to directly bind a 5'TOP sequence. Authors: Lahr, R.M. / Mack, S.M. / Heroux, A. / Blagden, S.P. / Bousquet-Antonelli, C. / Deragon, J.M. / Berman, A.J. #1:  Journal: To Be Published Journal: To Be PublishedTitle: The LARP1-specific domain DM15 repurposes HEAT-like repeats to directly bind messenger RNA Authors: Lahr, R.M. / Mack, S.M. / Heroux, A. / Blagden, S.P. / Bousquet-Antonelli, C. / Deragon, J.M. / Berman, A.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4zc4.cif.gz 4zc4.cif.gz | 146.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4zc4.ent.gz pdb4zc4.ent.gz | 116 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4zc4.json.gz 4zc4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zc/4zc4 https://data.pdbj.org/pub/pdb/validation_reports/zc/4zc4 ftp://data.pdbj.org/pub/pdb/validation_reports/zc/4zc4 ftp://data.pdbj.org/pub/pdb/validation_reports/zc/4zc4 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 19616.281 Da / Num. of mol.: 4 / Fragment: UNP residues 873-1023 Source method: isolated from a genetically manipulated source Details: QHPSHELLKENGFTQHVYHKYRRRCLNERKRLGIGQSQEMNTLFRFWSFFLRDHFNKKMYEEFKQLALEDAKEGYRYGLECLFRYYSYGLEKKFRLDIFKDFQEETVKDYEAGQLYGLEKFWAFLKYSKAKNLDIDPKLQEYLGKFR Source: (gene. exp.)   Homo sapiens (human) / Gene: LARP1, KIAA0731, LARP / Production host: Homo sapiens (human) / Gene: LARP1, KIAA0731, LARP / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q6PKG0 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: Q6PKG0#2: Chemical |  Sulfate Sulfate#3: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.41 Å3/Da / Density % sol: 49 % |
|---|---|
Crystal grow | Temperature: 304 K / Method: vapor diffusion, hanging drop / pH: 6.3 / Details: 0.225 M ammonium chloride, pH 6.3, 23% PEG 3350 / Temp details: Room Temp |
-Data collection
| Diffraction | Mean temperature: 80 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X25 / Wavelength: 1.1 Å / Beamline: X25 / Wavelength: 1.1 Å |
| Detector | Type: PSI PILATUS 6M / Detector: PIXEL / Date: Apr 16, 2013 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.1 Å / Relative weight: 1 : 1.1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.62→76.57 Å / Num. obs: 87993 / % possible obs: 95 % / Redundancy: 5.4 % / Rmerge(I) obs: 0.153 / Net I/σ(I): 34.582 |
| Reflection shell | Highest resolution: 1.62 Å / Redundancy: 2.4 % / Rmerge(I) obs: 0.108 / Mean I/σ(I) obs: 1.4 / % possible all: 63.66 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MAD / Resolution: 1.86→38.285 Å / SU ML: 0.2 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 24.54 / Stereochemistry target values: ML MAD / Resolution: 1.86→38.285 Å / SU ML: 0.2 / Cross valid method: FREE R-VALUE / σ(F): 1.34 / Phase error: 24.54 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.86→38.285 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj



