+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4yc5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Beta1 synthetic solenoid protein | |||||||||
 Components Components | beta1 | |||||||||
 Keywords Keywords |  STRUCTURAL PROTEIN / STRUCTURAL PROTEIN /  solenoid / solenoid /  scaffold scaffold | |||||||||
| Function / homology | E3 ubiquitin-protein ligase SopA / Pectate Lyase C-like / 3 Solenoid / Mainly Beta Function and homology information Function and homology information | |||||||||
| Biological species | synthetic construct (others) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MIR / Resolution: 1.755 Å MIR / Resolution: 1.755 Å | |||||||||
 Authors Authors | Murray, J.W. | |||||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| |||||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2016 Journal: Proc.Natl.Acad.Sci.USA / Year: 2016Title: Synthetic beta-solenoid proteins with the fragment-free computational design of a beta-hairpin extension. Authors: MacDonald, J.T. / Kabasakal, B.V. / Godding, D. / Kraatz, S. / Henderson, L. / Barber, J. / Freemont, P.S. / Murray, J.W. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4yc5.cif.gz 4yc5.cif.gz | 98.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4yc5.ent.gz pdb4yc5.ent.gz | 75.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4yc5.json.gz 4yc5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yc/4yc5 https://data.pdbj.org/pub/pdb/validation_reports/yc/4yc5 ftp://data.pdbj.org/pub/pdb/validation_reports/yc/4yc5 ftp://data.pdbj.org/pub/pdb/validation_reports/yc/4yc5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4ycqC  4ydtC  4yeiC  4yfoC  5di5C  5dn0C  5dnsC  5dqaC  5draC  5dzbC  3du1S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 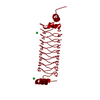
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 24676.037 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: synthetic protein sequence / Source: (gene. exp.) synthetic construct (others) / Production host:   Escherichia coli (E. coli) / Strain (production host): KRX Escherichia coli (E. coli) / Strain (production host): KRX | ||
|---|---|---|---|
| #2: Chemical |  Chloride Chloride#3: Water | ChemComp-HOH / |  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.39 Å3/Da / Density % sol: 63.69 % / Description: long rod |
|---|---|
Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop / pH: 5.5 / Details: 0.1 M Bis Tris, 3.0 M sodium chloride. / PH range: 5.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I02 / Wavelength: 1.0403 Å / Beamline: I02 / Wavelength: 1.0403 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Oct 28, 2010 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.0403 Å / Relative weight: 1 : 1.0403 Å / Relative weight: 1 |
| Reflection | Resolution: 1.75→64.57 Å / Num. all: 34852 / Num. obs: 34852 / % possible obs: 99.71 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 13.9 % / Rmerge(I) obs: 0.099 / Rsym value: 0.099 / Net I/av σ(I): 22.3587 / Net I/σ(I): 7.2971 |
| Reflection shell | Resolution: 1.75→1.8 Å / Redundancy: 14.24 % / Rmerge(I) obs: 0.83 / Mean I/σ(I) obs: 1.01 / % possible all: 99.85 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MIR MIRStarting model: 3DU1 Resolution: 1.755→64.57 Å / SU ML: 0.14 / Cross valid method: FREE R-VALUE / σ(F): 1.37 / Phase error: 16.67 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.755→64.57 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj


