[English] 日本語
 Yorodumi
Yorodumi- PDB-4tpu: CRYSTAL STRUCTURE OF FERREDOXIN-DEPENDENT DISULFIDE REDUCTASE FRO... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4tpu | ||||||
|---|---|---|---|---|---|---|---|
| Title | CRYSTAL STRUCTURE OF FERREDOXIN-DEPENDENT DISULFIDE REDUCTASE FROM METHANOSARCINA ACETIVORANS | ||||||
 Components Components | Rubredoxin | ||||||
 Keywords Keywords |  ELECTRON TRANSPORT / FTR-LIKE PROTEIN / DISULFIDE REDUCTASE ELECTRON TRANSPORT / FTR-LIKE PROTEIN / DISULFIDE REDUCTASE | ||||||
| Function / homology |  Function and homology information Function and homology information ferredoxin-thioredoxin reductase activity / ferredoxin-thioredoxin reductase activity /  ferredoxin:thioredoxin reductase / oxidoreductase activity, acting on iron-sulfur proteins as donors / 4 iron, 4 sulfur cluster binding / iron ion binding ferredoxin:thioredoxin reductase / oxidoreductase activity, acting on iron-sulfur proteins as donors / 4 iron, 4 sulfur cluster binding / iron ion bindingSimilarity search - Function | ||||||
| Biological species |   Methanosarcina acetivorans (archaea) Methanosarcina acetivorans (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 2.355 Å SAD / Resolution: 2.355 Å | ||||||
 Authors Authors | Kumar, A.K. / Yennawar, H.P. / Yennawar, N.H. / Ferry, J.G. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2015 Journal: Biochemistry / Year: 2015Title: Structural and Biochemical Characterization of a Ferredoxin:Thioredoxin Reductase-like Enzyme from Methanosarcina acetivorans. Authors: Kumar, A.K. / Kumar, R.S. / Yennawar, N.H. / Yennawar, H.P. / Ferry, J.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4tpu.cif.gz 4tpu.cif.gz | 117.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4tpu.ent.gz pdb4tpu.ent.gz | 91.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4tpu.json.gz 4tpu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tp/4tpu https://data.pdbj.org/pub/pdb/validation_reports/tp/4tpu ftp://data.pdbj.org/pub/pdb/validation_reports/tp/4tpu ftp://data.pdbj.org/pub/pdb/validation_reports/tp/4tpu | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein |  Mass: 20360.223 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Methanosarcina acetivorans (archaea) / Strain: ATCC 35395 / DSM 2834 / JCM 12185 / C2A / Gene: MA_1659 / Production host: Methanosarcina acetivorans (archaea) / Strain: ATCC 35395 / DSM 2834 / JCM 12185 / C2A / Gene: MA_1659 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q8TQ92 Escherichia coli (E. coli) / References: UniProt: Q8TQ92 |
|---|
-Non-polymers , 7 types, 63 molecules 


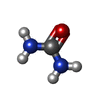









| #2: Chemical | ChemComp-SO4 /  Sulfate Sulfate | ||||||
|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-SF4 /  Iron–sulfur cluster Iron–sulfur cluster | ||||||
| #4: Chemical | ChemComp-FE /  Iron Iron | ||||||
| #5: Chemical |  Urea Urea#6: Chemical | ChemComp-NA / #7: Chemical | ChemComp-BR /  Bromide Bromide#8: Water | ChemComp-HOH / |  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.31 Å3/Da / Density % sol: 62.86 % |
|---|---|
Crystal grow | Temperature: 294 K / Method: vapor diffusion, sitting drop / pH: 7.5 Details: 2M AMMONIUM SULFATE, 0.1M HEPES BUFFER, 0.1M UREA, PH 7.5, VAPOR DIFFUSION, SITTING DROP, TEMPERATURE 294K |
-Data collection
| Diffraction | Mean temperature: 93 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CHESS CHESS  / Beamline: F2 / Wavelength: 0.918 Å / Beamline: F2 / Wavelength: 0.918 Å |
| Detector | Type: ADSC QUANTUM 270 / Detector: CCD / Date: Jun 3, 2011 |
| Radiation | Monochromator: SI 111 CHANNEL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.918 Å / Relative weight: 1 : 0.918 Å / Relative weight: 1 |
| Reflection | Resolution: 2.355→50 Å / Num. all: 11465 / Num. obs: 11465 / % possible obs: 99.9 % / Observed criterion σ(I): 1 / Redundancy: 18.3 % / Rmerge(I) obs: 0.11 / Net I/σ(I): 23.9 |
| Reflection shell | Resolution: 2.35→2.39 Å / Redundancy: 18.6 % / Mean I/σ(I) obs: 1.6 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  SAD / Resolution: 2.355→29.47 Å / SU ML: 0.64 / Cross valid method: FREE R-VALUE / σ(F): 0 / Phase error: 25.72 / Stereochemistry target values: ML SAD / Resolution: 2.355→29.47 Å / SU ML: 0.64 / Cross valid method: FREE R-VALUE / σ(F): 0 / Phase error: 25.72 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.89 Å / VDW probe radii: 1 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 47.69 Å2 / ksol: 0.41 e/Å3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.355→29.47 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 22.2073 Å / Origin y: 18.3517 Å / Origin z: 4.8343 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller








 PDBj
PDBj








