[English] 日本語
 Yorodumi
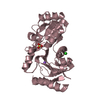
Yorodumi- PDB-4pp9: ITK kinase domain with compound 1 (N-[1-(3-CYANOBENZYL)-1H-PYRAZO... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4pp9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | ITK kinase domain with compound 1 (N-[1-(3-CYANOBENZYL)-1H-PYRAZOL-4-YL]-2H-INDAZOLE-3-CARBOXAMIDE) | ||||||
 Components Components | Tyrosine-protein kinase ITK/TSK | ||||||
 Keywords Keywords | transferase/transferase inhibitor /  protein kinase / phospho-transfer / transferase-transferase inhibitor complex protein kinase / phospho-transfer / transferase-transferase inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationgamma-delta T cell activation / NK T cell differentiation / activation of phospholipase C activity / Generation of second messenger molecules / cellular defense response /  T cell activation / FCERI mediated Ca+2 mobilization / positive regulation of cytokine production / B cell receptor signaling pathway / T cell activation / FCERI mediated Ca+2 mobilization / positive regulation of cytokine production / B cell receptor signaling pathway /  non-specific protein-tyrosine kinase ...gamma-delta T cell activation / NK T cell differentiation / activation of phospholipase C activity / Generation of second messenger molecules / cellular defense response / non-specific protein-tyrosine kinase ...gamma-delta T cell activation / NK T cell differentiation / activation of phospholipase C activity / Generation of second messenger molecules / cellular defense response /  T cell activation / FCERI mediated Ca+2 mobilization / positive regulation of cytokine production / B cell receptor signaling pathway / T cell activation / FCERI mediated Ca+2 mobilization / positive regulation of cytokine production / B cell receptor signaling pathway /  non-specific protein-tyrosine kinase / non-membrane spanning protein tyrosine kinase activity / cell-cell junction / T cell receptor signaling pathway / non-specific protein-tyrosine kinase / non-membrane spanning protein tyrosine kinase activity / cell-cell junction / T cell receptor signaling pathway /  adaptive immune response / intracellular signal transduction / adaptive immune response / intracellular signal transduction /  phosphorylation / phosphorylation /  signal transduction / signal transduction /  ATP binding / ATP binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.58 Å MOLECULAR REPLACEMENT / Resolution: 2.58 Å | ||||||
 Authors Authors | Eigenbrot, C. / Shia, S. | ||||||
 Citation Citation |  Journal: Bioorg.Med.Chem.Lett. / Year: 2014 Journal: Bioorg.Med.Chem.Lett. / Year: 2014Title: Discovery and optimization of indazoles as potent and selective interleukin-2 inducible T cell kinase (ITK) inhibitors. Authors: Pastor, R.M. / Burch, J.D. / Magnuson, S. / Ortwine, D.F. / Chen, Y. / De La Torre, K. / Ding, X. / Eigenbrot, C. / Johnson, A. / Liimatta, M. / Liu, Y. / Shia, S. / Wang, X. / Wu, L.C. / Pei, Z. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4pp9.cif.gz 4pp9.cif.gz | 210.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4pp9.ent.gz pdb4pp9.ent.gz | 169.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4pp9.json.gz 4pp9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/pp/4pp9 https://data.pdbj.org/pub/pdb/validation_reports/pp/4pp9 ftp://data.pdbj.org/pub/pdb/validation_reports/pp/4pp9 ftp://data.pdbj.org/pub/pdb/validation_reports/pp/4pp9 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4ppaC  4ppbC  4ppcC  1sm2S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 30214.492 Da / Num. of mol.: 2 / Fragment: kinase domain / Mutation: Y512E Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: ITK, EMT, LYK / Production host: Homo sapiens (human) / Gene: ITK, EMT, LYK / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm)References: UniProt: Q08881,  non-specific protein-tyrosine kinase non-specific protein-tyrosine kinase#2: Chemical |  Sulfate Sulfate#3: Chemical | #4: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.55 Å3/Da / Density % sol: 51.76 % |
|---|---|
Crystal grow | Temperature: 280 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: sodium nitrate, PEG3350, pH 8.5, VAPOR DIFFUSION, SITTING DROP, temperature 380K |
-Data collection
| Diffraction | Mean temperature: 110 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.2 / Wavelength: 1 Å / Beamline: 5.0.2 / Wavelength: 1 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Jun 30, 2010 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.6→40 Å / Num. all: 17994 / Num. obs: 17975 / % possible obs: 92.6 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Redundancy: 3.4 % / Biso Wilson estimate: 48.64 Å2 / Rsym value: 0.148 / Net I/σ(I): 7.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1SM2 Resolution: 2.58→25.34 Å / Cor.coef. Fo:Fc: 0.9064 / Cor.coef. Fo:Fc free: 0.8591 / SU R Cruickshank DPI: 0.903 Isotropic thermal model: individual atomic plus TLS in two groups Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 44.32 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.4 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.58→25.34 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.58→2.74 Å / Total num. of bins used: 9
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj