+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4pol | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structures of thioredoxin with mesna at 2.8A resolution | ||||||
 Components Components | Thioredoxin | ||||||
 Keywords Keywords |  OXIDOREDUCTASE OXIDOREDUCTASE | ||||||
| Function / homology |  Function and homology information Function and homology informationProtein repair / cellular detoxification of hydrogen peroxide / positive regulation of peptidyl-cysteine S-nitrosylation / protein-disulfide reductase (NAD(P)H) activity / thioredoxin-disulfide reductase (NADPH) activity / Interconversion of nucleotide di- and triphosphates / negative regulation of protein export from nucleus / Regulation of FOXO transcriptional activity by acetylation / NFE2L2 regulating anti-oxidant/detoxification enzymes / response to nitric oxide ...Protein repair / cellular detoxification of hydrogen peroxide / positive regulation of peptidyl-cysteine S-nitrosylation / protein-disulfide reductase (NAD(P)H) activity / thioredoxin-disulfide reductase (NADPH) activity / Interconversion of nucleotide di- and triphosphates / negative regulation of protein export from nucleus / Regulation of FOXO transcriptional activity by acetylation / NFE2L2 regulating anti-oxidant/detoxification enzymes / response to nitric oxide / Detoxification of Reactive Oxygen Species / The NLRP3 inflammasome /  protein-disulfide reductase activity / positive regulation of DNA binding / protein-disulfide reductase activity / positive regulation of DNA binding /  Purinergic signaling in leishmaniasis infection / activation of protein kinase B activity / cell redox homeostasis / TP53 Regulates Metabolic Genes / response to radiation / positive regulation of peptidyl-serine phosphorylation / Oxidative Stress Induced Senescence / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / negative regulation of transcription by RNA polymerase II / protein homodimerization activity / Purinergic signaling in leishmaniasis infection / activation of protein kinase B activity / cell redox homeostasis / TP53 Regulates Metabolic Genes / response to radiation / positive regulation of peptidyl-serine phosphorylation / Oxidative Stress Induced Senescence / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / negative regulation of transcription by RNA polymerase II / protein homodimerization activity /  RNA binding / extracellular exosome / extracellular region / RNA binding / extracellular exosome / extracellular region /  nucleoplasm / nucleoplasm /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.8 Å MOLECULAR REPLACEMENT / Resolution: 2.8 Å | ||||||
 Authors Authors | Sridhar, V. / Chie-Leon, B. / Badger, J. / Nienaber, V.L. / Hausheer, F.H. | ||||||
 Citation Citation |  Journal: J Pharmacol Clin Toxicol / Year: 2014 Journal: J Pharmacol Clin Toxicol / Year: 2014Title: BNP7787 Forms Novel Covalent Adducts on Human Thioredoxin and Modulates Thioredoxin Activity Authors: Parker, A.R. / Nienaber, V.L. / Petluru, P.N. / Sridhar, V. / Leverett, B.D. / Ayala, P.Y. / Zhao, M. / Chie-Leon, B. / Jair, K. / Kochat, H. / Badger, J. / Hausheer, F.H. | ||||||
| History |
|
- Structure visualization
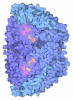
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4pol.cif.gz 4pol.cif.gz | 91.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4pol.ent.gz pdb4pol.ent.gz | 70.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4pol.json.gz 4pol.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/po/4pol https://data.pdbj.org/pub/pdb/validation_reports/po/4pol ftp://data.pdbj.org/pub/pdb/validation_reports/po/4pol ftp://data.pdbj.org/pub/pdb/validation_reports/po/4pol | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4pokC  4pomC  4po4S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| 5 | 
| ||||||||
| 6 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein |  / Trx / ATL-derived factor / ADF / Surface-associated sulphydryl protein / SASP / Trx / ATL-derived factor / ADF / Surface-associated sulphydryl protein / SASPMass: 12051.055 Da / Num. of mol.: 4 / Mutation: E13K, D16K, E95K, E103K Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: TXN, TRDX, TRX, TRX1 / Production host: Homo sapiens (human) / Gene: TXN, TRDX, TRX, TRX1 / Production host:   Escherichia coli (E. coli) / References: UniProt: P10599 Escherichia coli (E. coli) / References: UniProt: P10599#2: Chemical | ChemComp-COM / |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.44 Å3/Da / Density % sol: 49.52 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 7 Details: 20% PEG3350, 0.2 M KCl, 60 mg/mL Trx protein, pH 7.0, VAPOR DIFFUSION, SITTING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 5.0.1 / Wavelength: 0.9774 Å / Beamline: 5.0.1 / Wavelength: 0.9774 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: May 24, 2011 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9774 Å / Relative weight: 1 : 0.9774 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→48 Å / Num. all: 11124 / Num. obs: 11124 / % possible obs: 96.1 % / Redundancy: 2.9 % / Rmerge(I) obs: 0.095 / Net I/σ(I): 8.5 |
| Reflection shell | Resolution: 2.8→2.95 Å / Redundancy: 2.4 % / Rmerge(I) obs: 0.396 / Mean I/σ(I) obs: 2.2 / Num. unique all: 1533 / % possible all: 92.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4PO4 Resolution: 2.8→47.95 Å / Cor.coef. Fo:Fc: 0.888 / Cor.coef. Fo:Fc free: 0.827 / SU B: 18.543 / SU ML: 0.376 / Cross valid method: THROUGHOUT / ESU R Free: 0.497 / Stereochemistry target values: MAXIMUM LIKELIHOOD
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 27.592 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.8→47.95 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.8→2.873 Å / Total num. of bins used: 20 /
|
 Movie
Movie Controller
Controller











 PDBj
PDBj