| Entry | Database: PDB / ID: 4oxm
|
|---|
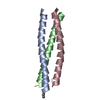
| Title | CRYSTAL STRUCTURE OF Central Coiled-Coil from Influenza Hemagglutinin HA2 without Heptad Repeat Stutter |
|---|
 Components Components | HA2-Del |
|---|
 Keywords Keywords |  VIRAL PROTEIN / VIRAL PROTEIN /  coiled-coil / coiled-coil /  Influenza / Influenza /  Hemagglutinin / Hemagglutinin /  Stutter Stutter |
|---|
| Function / homology |  Haemagglutinin, influenzavirus B / Haemagglutinin, influenzavirus B /  Haemagglutinin / Haemagglutinin /  Haemagglutinin, influenzavirus A/B / host cell surface receptor binding / fusion of virus membrane with host plasma membrane / Haemagglutinin, influenzavirus A/B / host cell surface receptor binding / fusion of virus membrane with host plasma membrane /  viral envelope / Truncated hemagglutinin viral envelope / Truncated hemagglutinin Function and homology information Function and homology information |
|---|
| Biological species |   unidentified influenza virus unidentified influenza virus |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.9 Å molecular replacement / Resolution: 1.9 Å |
|---|
 Authors Authors | Malashkevich, V.N. / Higgins, C.D. / Lai, J.R. / Almo, S.C. |
|---|
 Citation Citation |  Journal: to be published Journal: to be published
Title: CRYSTAL STRUCTURE OF Central Coiled-Coil from InfluenzaHemagglutinin HA2 without Heptad Repeat Stutter
Authors: Malashkevich, V.N. / Higgins, C.D. / Lai, J.R. / Almo, S.C. |
|---|
| History | | Deposition | Feb 5, 2014 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Apr 30, 2014 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Dec 24, 2014 | Group: Database references |
|---|
| Revision 1.2 | Nov 22, 2017 | Group: Database references / Derived calculations ...Database references / Derived calculations / Other / Refinement description / Source and taxonomy
Category: citation / pdbx_database_status ...citation / pdbx_database_status / pdbx_entity_src_syn / pdbx_struct_assembly / pdbx_struct_oper_list / software
Item: _citation.journal_id_CSD / _pdbx_database_status.pdb_format_compatible ..._citation.journal_id_CSD / _pdbx_database_status.pdb_format_compatible / _pdbx_entity_src_syn.pdbx_alt_source_flag / _pdbx_struct_assembly.oligomeric_details / _pdbx_struct_oper_list.symmetry_operation |
|---|
| Revision 1.3 | Dec 27, 2023 | Group: Data collection / Database references / Refinement description
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / refine_hist / struct_ncs_dom_lim
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _refine_hist.d_res_low / _refine_hist.number_atoms_solvent / _refine_hist.number_atoms_total / _refine_hist.pdbx_number_atoms_ligand / _refine_hist.pdbx_number_atoms_nucleic_acid / _refine_hist.pdbx_number_atoms_protein / _struct_ncs_dom_lim.beg_auth_comp_id / _struct_ncs_dom_lim.beg_label_asym_id / _struct_ncs_dom_lim.beg_label_comp_id / _struct_ncs_dom_lim.beg_label_seq_id / _struct_ncs_dom_lim.end_auth_comp_id / _struct_ncs_dom_lim.end_label_asym_id / _struct_ncs_dom_lim.end_label_comp_id / _struct_ncs_dom_lim.end_label_seq_id |
|---|
| Revision 1.4 | Mar 27, 2024 | Group: Refinement description / Category: pdbx_initial_refinement_model |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords VIRAL PROTEIN /
VIRAL PROTEIN /  coiled-coil /
coiled-coil /  Influenza /
Influenza /  Hemagglutinin /
Hemagglutinin /  Stutter
Stutter Haemagglutinin, influenzavirus B /
Haemagglutinin, influenzavirus B /  Haemagglutinin /
Haemagglutinin /  Haemagglutinin, influenzavirus A/B / host cell surface receptor binding / fusion of virus membrane with host plasma membrane /
Haemagglutinin, influenzavirus A/B / host cell surface receptor binding / fusion of virus membrane with host plasma membrane /  viral envelope / Truncated hemagglutinin
viral envelope / Truncated hemagglutinin Function and homology information
Function and homology information
 unidentified influenza virus
unidentified influenza virus X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.9 Å
molecular replacement / Resolution: 1.9 Å  Authors
Authors Citation
Citation Journal: to be published
Journal: to be published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4oxm.cif.gz
4oxm.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4oxm.ent.gz
pdb4oxm.ent.gz PDB format
PDB format 4oxm.json.gz
4oxm.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ox/4oxm
https://data.pdbj.org/pub/pdb/validation_reports/ox/4oxm ftp://data.pdbj.org/pub/pdb/validation_reports/ox/4oxm
ftp://data.pdbj.org/pub/pdb/validation_reports/ox/4oxm Links
Links Assembly
Assembly
 Components
Components
 unidentified influenza virus / References: UniProt: A8TXX2*PLUS
unidentified influenza virus / References: UniProt: A8TXX2*PLUS Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 31-ID / Wavelength: 0.9791 Å
/ Beamline: 31-ID / Wavelength: 0.9791 Å : 0.9791 Å / Relative weight: 1
: 0.9791 Å / Relative weight: 1 
 molecular replacement
molecular replacement Processing
Processing :
:  MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller








 PDBj
PDBj




