[English] 日本語
 Yorodumi
Yorodumi- PDB-4l68: Structure of the psedudokinase domain of BIR2, an immune regulato... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4l68 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of the psedudokinase domain of BIR2, an immune regulator of the RLK/Pelle family | ||||||
 Components Components | Leucine-rich repeat protein kinase-like protein | ||||||
 Keywords Keywords |  SIGNALING PROTEIN / SIGNALING PROTEIN /  pseudokinase / negative immune regulator pseudokinase / negative immune regulator | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of defense response to fungus / negative regulation of defense response to bacterium /  chloroplast / defense response / chloroplast / defense response /  nucleotide binding / nucleotide binding /  plasma membrane / plasma membrane /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Arabidopsis thaliana (thale cress) Arabidopsis thaliana (thale cress) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | ||||||
 Authors Authors | Blaum, B.S. / Stehle, T. | ||||||
 Citation Citation |  Journal: J.Struct.Biol. / Year: 2014 Journal: J.Struct.Biol. / Year: 2014Title: Structure of the pseudokinase domain of BIR2, a regulator of BAK1-mediated immune signaling in Arabidopsis. Authors: Blaum, B.S. / Mazzotta, S. / Noldeke, E.R. / Halter, T. / Madlung, J. / Kemmerling, B. / Stehle, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4l68.cif.gz 4l68.cif.gz | 270.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4l68.ent.gz pdb4l68.ent.gz | 220.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4l68.json.gz 4l68.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/l6/4l68 https://data.pdbj.org/pub/pdb/validation_reports/l6/4l68 ftp://data.pdbj.org/pub/pdb/validation_reports/l6/4l68 ftp://data.pdbj.org/pub/pdb/validation_reports/l6/4l68 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| 2 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
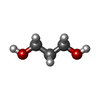
| #1: Protein |  / BIR2 / Leucine-rich repeat receptor-like protein kinase / Putative receptor kinase / Receptor-like ...BIR2 / Leucine-rich repeat receptor-like protein kinase / Putative receptor kinase / Receptor-like protein kinase-like protein / BIR2 / Leucine-rich repeat receptor-like protein kinase / Putative receptor kinase / Receptor-like ...BIR2 / Leucine-rich repeat receptor-like protein kinase / Putative receptor kinase / Receptor-like protein kinase-like proteinMass: 37869.934 Da / Num. of mol.: 2 / Fragment: cytosolic domain (UNP residues 271-605) Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Arabidopsis thaliana (thale cress) / Gene: AT3G28450, At3g28450/MFJ20_13, LRR-RLK / Production host: Arabidopsis thaliana (thale cress) / Gene: AT3G28450, At3g28450/MFJ20_13, LRR-RLK / Production host:   Escherichia coli (E. coli) / References: UniProt: Q9LSI9 Escherichia coli (E. coli) / References: UniProt: Q9LSI9#2: Chemical | ChemComp-PDO / |  1,3-Propanediol 1,3-Propanediol#3: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.92 Å3/Da / Density % sol: 57.83 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: 0.03 M n-ethylene glycols, 0.1 M bicine/Trizma base, 10% PEG20000, 20% PEG550 MME, pH 8.5, VAPOR DIFFUSION, SITTING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06DA / Wavelength: 1.07 Å / Beamline: X06DA / Wavelength: 1.07 Å |
| Detector | Type: DECTRIS PILATUS 2M-F / Detector: PIXEL / Date: Apr 30, 2012 |
| Radiation | Monochromator: Bartels DCCM Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.07 Å / Relative weight: 1 : 1.07 Å / Relative weight: 1 |
| Reflection | Resolution: 2→48.122 Å / Num. all: 60598 / Num. obs: 60269 / % possible obs: 100 % / Observed criterion σ(F): 3 / Observed criterion σ(I): 3 / Biso Wilson estimate: 33.8 Å2 |
| Reflection shell | Resolution: 2→2.19 Å / % possible all: 23.52 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 2→48.122 Å / SU ML: 0.2 / σ(F): 2 / Phase error: 20.42 / Stereochemistry target values: ML MOLECULAR REPLACEMENT / Resolution: 2→48.122 Å / SU ML: 0.2 / σ(F): 2 / Phase error: 20.42 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→48.122 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 22 / % reflection obs: 100 %
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj