+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4hpq | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of the Atg17-Atg31-Atg29 Complex | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  PROTEIN TRANSPORT / PROTEIN TRANSPORT /  Autophagy Autophagy | ||||||
| Function / homology |  Function and homology information Function and homology informationAtg1/ULK1 kinase complex / phagophore assembly site membrane / piecemeal microautophagy of the nucleus / phagophore assembly site / protein kinase activator activity /  autophagosome assembly / autophagosome assembly /  autophagy / autophagy /  protein transport / molecular adaptor activity protein transport / molecular adaptor activitySimilarity search - Function | ||||||
| Biological species |   Lachancea thermotolerans CBS 6340 (fungus) Lachancea thermotolerans CBS 6340 (fungus) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 3.06 Å SAD / Resolution: 3.06 Å | ||||||
 Authors Authors | Stanley, R.E. / Ragusa, M.J. / Hurley, J.H. | ||||||
 Citation Citation |  Journal: Cell(Cambridge,Mass.) / Year: 2012 Journal: Cell(Cambridge,Mass.) / Year: 2012Title: Architecture of the atg17 complex as a scaffold for autophagosome biogenesis. Authors: Ragusa, M.J. / Stanley, R.E. / Hurley, J.H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4hpq.cif.gz 4hpq.cif.gz | 237 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4hpq.ent.gz pdb4hpq.ent.gz | 192.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4hpq.json.gz 4hpq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hp/4hpq https://data.pdbj.org/pub/pdb/validation_reports/hp/4hpq ftp://data.pdbj.org/pub/pdb/validation_reports/hp/4hpq ftp://data.pdbj.org/pub/pdb/validation_reports/hp/4hpq | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 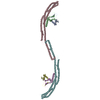
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
| #1: Protein | Mass: 7222.390 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Lachancea thermotolerans CBS 6340 (fungus) Lachancea thermotolerans CBS 6340 (fungus)Strain: ATCC 56472 / CBS 6340 / NRRL Y-8284 / Plasmid: pst39 / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: C5DF24 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: C5DF24#2: Protein | Mass: 18288.232 Da / Num. of mol.: 2 / Mutation: L87M, L110M Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Lachancea thermotolerans CBS 6340 (fungus) Lachancea thermotolerans CBS 6340 (fungus)Strain: ATCC 56472 / CBS 6340 / NRRL Y-8284 / Plasmid: pst39 / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: C5DEB9 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: C5DEB9#3: Protein | Mass: 48312.809 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Lachancea thermotolerans CBS 6340 (fungus) Lachancea thermotolerans CBS 6340 (fungus)Strain: ATCC 56472 / CBS 6340 / NRRL Y-8284 / Plasmid: pst39 / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: C5DFJ6 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: C5DFJ6Sequence details | THE COMPLETE CRYSTALLIZED SEQUENCE OF CHAIN A AND D IS: ...THE COMPLETE CRYSTALLIZ | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 5.41 Å3/Da / Density % sol: 77.24 % |
|---|---|
Crystal grow | Temperature: 294 K / Method: vapor diffusion, hanging drop / pH: 8 Details: 50 mM Tris pH8, 4-10% peg 2KMME, 10-20% ethylene glycol, 100 mM NaCL, pH 8.0, VAPOR DIFFUSION, HANGING DROP, temperature 294K |
-Data collection
| Diffraction |
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| ||||||||||||||||||
| Detector |
| ||||||||||||||||||
| Radiation |
| ||||||||||||||||||
| Radiation wavelength | Relative weight: 1 | ||||||||||||||||||
| Reflection | Resolution: 3.055→50 Å / Num. all: 59513 / Num. obs: 47730 / % possible obs: 80.2 % / Observed criterion σ(F): 2 / Observed criterion σ(I): 1 / Redundancy: 5.9 % / Rmerge(I) obs: 0.096 / Rsym value: 0.074 / Net I/σ(I): 16 | ||||||||||||||||||
| Reflection shell | Resolution: 3.055→3.2 Å / Rmerge(I) obs: 0.254 / Mean I/σ(I) obs: 4.1 / % possible all: 29 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  SAD / Resolution: 3.06→46.56 Å / Cor.coef. Fo:Fc: 0.851 / Cor.coef. Fo:Fc free: 0.812 / SU B: 20.473 / SU ML: 0.377 / Cross valid method: THROUGHOUT / ESU R: 1.224 / ESU R Free: 0.529 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS SAD / Resolution: 3.06→46.56 Å / Cor.coef. Fo:Fc: 0.851 / Cor.coef. Fo:Fc free: 0.812 / SU B: 20.473 / SU ML: 0.377 / Cross valid method: THROUGHOUT / ESU R: 1.224 / ESU R Free: 0.529 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 71.58 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.06→46.56 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.056→3.135 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller








 PDBj
PDBj

