+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4guk | ||||||
|---|---|---|---|---|---|---|---|
| Title | New crystal form structure of human NCS1 | ||||||
 Components Components | Neuronal calcium sensor 1 | ||||||
 Keywords Keywords |  PROTEIN BINDING / alpha / PROTEIN BINDING / alpha /  neuronal calcium sensor neuronal calcium sensor | ||||||
| Function / homology |  Function and homology information Function and homology informationcalcium sensitive guanylate cyclase activator activity / regulation of neuron projection development /  voltage-gated calcium channel activity / voltage-gated calcium channel activity /  postsynaptic density / postsynaptic density /  axon / intracellular membrane-bounded organelle / axon / intracellular membrane-bounded organelle /  dendrite / dendrite /  calcium ion binding / perinuclear region of cytoplasm / calcium ion binding / perinuclear region of cytoplasm /  Golgi apparatus ...calcium sensitive guanylate cyclase activator activity / regulation of neuron projection development / Golgi apparatus ...calcium sensitive guanylate cyclase activator activity / regulation of neuron projection development /  voltage-gated calcium channel activity / voltage-gated calcium channel activity /  postsynaptic density / postsynaptic density /  axon / intracellular membrane-bounded organelle / axon / intracellular membrane-bounded organelle /  dendrite / dendrite /  calcium ion binding / perinuclear region of cytoplasm / calcium ion binding / perinuclear region of cytoplasm /  Golgi apparatus / Golgi apparatus /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.75 Å MOLECULAR REPLACEMENT / Resolution: 1.75 Å | ||||||
 Authors Authors | Fan, C. / Lolis, E. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: new crystal form structure of human NCS1 Authors: Fan, C. / Lolis, E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4guk.cif.gz 4guk.cif.gz | 318.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4guk.ent.gz pdb4guk.ent.gz | 255.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4guk.json.gz 4guk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gu/4guk https://data.pdbj.org/pub/pdb/validation_reports/gu/4guk ftp://data.pdbj.org/pub/pdb/validation_reports/gu/4guk ftp://data.pdbj.org/pub/pdb/validation_reports/gu/4guk | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| 4 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 4 molecules ACBD
| #1: Protein |  / NCS-1 / Frequenin homolog / Frequenin-like protein / Frequenin-like ubiquitous protein / NCS-1 / Frequenin homolog / Frequenin-like protein / Frequenin-like ubiquitous proteinMass: 21902.668 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Details: 0.1mMIPTG 16C induction / Source: (gene. exp.)   Homo sapiens (human) / Strain: human Homo sapiens (human) / Strain: human / Gene: FLUP, FREQ, NCS1 / Plasmid: pET21a(+) / Production host: / Gene: FLUP, FREQ, NCS1 / Plasmid: pET21a(+) / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: P62166 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) / References: UniProt: P62166 |
|---|
-Non-polymers , 8 types, 408 molecules 



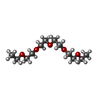










| #2: Chemical | ChemComp-PG4 /  Polyethylene glycol Polyethylene glycol#3: Chemical | ChemComp-EDO /  Ethylene glycol Ethylene glycol#4: Chemical | ChemComp-CA / #5: Chemical | ChemComp-NA / #6: Chemical | ChemComp-P3G / | #7: Chemical | ChemComp-P2G / ( #8: Chemical | ChemComp-ALA / |  Alanine Alanine#9: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.13 Å3/Da / Density % sol: 42.29 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: pH6.5, 0.2M NaAC, 30%PEG8000, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X25 / Wavelength: 1.1 Å / Beamline: X25 / Wavelength: 1.1 Å |
| Detector | Type: PSI PILATUS 6M / Detector: PIXEL / Date: Apr 28, 2011 |
| Radiation | Monochromator: graphite / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.1 Å / Relative weight: 1 : 1.1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.75→50 Å / Num. all: 75302 / Num. obs: 73721 / % possible obs: 97.9 % / Observed criterion σ(F): 2.7 / Observed criterion σ(I): 2.7 / Redundancy: 7.3 % / Rmerge(I) obs: 0.091 / Rsym value: 0.091 / Net I/σ(I): 11 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: human NCS1 Resolution: 1.75→50 Å / Isotropic thermal model: isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.75→50 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj






