[English] 日本語
 Yorodumi
Yorodumi- PDB-4c1g: Crystal structure of the metallo-beta-lactamase IMP-1 with D-captopril -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4c1g | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the metallo-beta-lactamase IMP-1 with D-captopril | ||||||
 Components Components | BETA-LACTAMASE IMP-1 | ||||||
 Keywords Keywords |  HYDROLASE / HYDROLASE /  ANTIBIOTIC RESISTANCE ANTIBIOTIC RESISTANCE | ||||||
| Function / homology |  Function and homology information Function and homology informationantibiotic catabolic process /  beta-lactamase activity / beta-lactamase activity /  beta-lactamase / beta-lactamase /  periplasmic space / response to antibiotic / zinc ion binding periplasmic space / response to antibiotic / zinc ion bindingSimilarity search - Function | ||||||
| Biological species |   SERRATIA MARCESCENS (bacteria) SERRATIA MARCESCENS (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.714 Å MOLECULAR REPLACEMENT / Resolution: 1.714 Å | ||||||
 Authors Authors | Zollman, D. / Brem, J. / McDonough, M.A. / van Berkel, S.S. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: Antimicrob. Agents Chemother. / Year: 2015 Journal: Antimicrob. Agents Chemother. / Year: 2015Title: Structural Basis of Metallo-beta-Lactamase Inhibition by Captopril Stereoisomers. Authors: Brem, J. / van Berkel, S.S. / Zollman, D. / Lee, S.Y. / Gileadi, O. / McHugh, P.J. / Walsh, T.R. / McDonough, M.A. / Schofield, C.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4c1g.cif.gz 4c1g.cif.gz | 190.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4c1g.ent.gz pdb4c1g.ent.gz | 149.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4c1g.json.gz 4c1g.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c1/4c1g https://data.pdbj.org/pub/pdb/validation_reports/c1/4c1g ftp://data.pdbj.org/pub/pdb/validation_reports/c1/4c1g ftp://data.pdbj.org/pub/pdb/validation_reports/c1/4c1g | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4bz3C  4c09C  4c1cC  4c1dC  4c1eC  4c1fC  4c1hC  1dd6S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 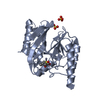
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 25146.676 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   SERRATIA MARCESCENS (bacteria) / Description: PLASMID DERIVED NON-GENOMIC. / Production host: SERRATIA MARCESCENS (bacteria) / Description: PLASMID DERIVED NON-GENOMIC. / Production host:   ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / Variant (production host): PLYSS / References: UniProt: P52699, ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) / Variant (production host): PLYSS / References: UniProt: P52699,  beta-lactamase beta-lactamase#2: Chemical | ChemComp-ZN / #3: Chemical | ChemComp-MCO / | #4: Chemical |  Sulfate Sulfate#5: Water | ChemComp-HOH / |  Water WaterNonpolymer details | ((S)-3-MERCAPTO-2-METHYLPROP | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.32 Å3/Da / Density % sol: 47 % / Description: NONE |
|---|---|
Crystal grow | pH: 4.5 Details: 0.05 M LI2SO4, 0.1 M SODIUM ACETATE, PH 4.5, 23 % W/V PEG 8000, 1MM DTT. |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.9795 / Beamline: I04 / Wavelength: 0.9795 |
| Detector | Type: DECTRIS PILATUS / Detector: PIXEL / Date: Jul 6, 2012 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9795 Å / Relative weight: 1 : 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 1.71→64.86 Å / Num. obs: 58579 / % possible obs: 100 % / Observed criterion σ(I): 0 / Redundancy: 9.5 % / Biso Wilson estimate: 22.06 Å2 / Rmerge(I) obs: 0.09 / Net I/σ(I): 13.7 |
| Reflection shell | Resolution: 1.71→1.76 Å / Redundancy: 10.1 % / Rmerge(I) obs: 0.63 / Mean I/σ(I) obs: 3.2 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1DD6 Resolution: 1.714→52.543 Å / SU ML: 0.17 / σ(F): 1.33 / Phase error: 15.69 / Stereochemistry target values: ML Details: CHAIN B MUCH MORE DISORDERED THAN CHAIN A SO INHIBITOR ONLY SEEN IN A. CONFORMATION OF RAMACHANDRAN OUTLIERS 59 ASN AND 66 ASP VALIDATED BY CLEAR ELECTRON DENSITY IN CHAIN A.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 49.19 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.714→52.543 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj