| Entry | Database: PDB / ID: 4b7r
|
|---|
| Title | H1N1 2009 Pandemic Influenza Virus: Resistance of the I223R Neuraminidase Mutant Explained by Kinetic and Structural Analysis |
|---|
 Components Components | NEURAMINIDASE |
|---|
 Keywords Keywords |  HYDROLASE / HYDROLASE /  NEURAMINIDASE INHIBITOR / NAI / NEURAMINIDASE INHIBITOR / NAI /  NAIS / NAIS /  ZANAMIVIR / RESISTANCE / ANTIVIRAL RESISTANCE ZANAMIVIR / RESISTANCE / ANTIVIRAL RESISTANCE |
|---|
| Function / homology |  Function and homology information Function and homology information |
|---|
| Biological species |    INFLUENZA A VIRUS INFLUENZA A VIRUS |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å MOLECULAR REPLACEMENT / Resolution: 1.9 Å |
|---|
 Authors Authors | Liu, J. / van der Vries, E. / Vachieri, S.G. / Xiong, X. / Collins, P.J. / Walker, P.A. / Haire, L.F. / Hay, A.J. / Schutten, M. / Osterhaus, A.D.M.E. ...Liu, J. / van der Vries, E. / Vachieri, S.G. / Xiong, X. / Collins, P.J. / Walker, P.A. / Haire, L.F. / Hay, A.J. / Schutten, M. / Osterhaus, A.D.M.E. / Martin, S.R. / Boucher, C.A.B. / Skehel, J.J. / Gamblin, S.J. |
|---|
 Citation Citation |  Journal: Plos Pathog. / Year: 2012 Journal: Plos Pathog. / Year: 2012
Title: H1N1 2009 Pandemic Influenza Virus: Resistance of the I223R Neuraminidase Mutant Explained by Kinetic and Structural Analysis
Authors: Van Der Vries, E. / Collins, P.J. / Vachieri, S.G. / Xiong, X. / Liu, J. / Walker, P.A. / Haire, L.F. / Hay, A.J. / Schutten, M. / Osterhaus, A.D.M.E. / Martin, S.R. / Boucher, C.A.B. / ...Authors: Van Der Vries, E. / Collins, P.J. / Vachieri, S.G. / Xiong, X. / Liu, J. / Walker, P.A. / Haire, L.F. / Hay, A.J. / Schutten, M. / Osterhaus, A.D.M.E. / Martin, S.R. / Boucher, C.A.B. / Skehel, J.J. / Gamblin, S.J. |
|---|
| History | | Deposition | Aug 21, 2012 | Deposition site: PDBE / Processing site: PDBE |
|---|
| Revision 1.0 | Oct 3, 2012 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Oct 10, 2012 | Group: Database references |
|---|
| Revision 1.2 | Jul 17, 2013 | Group: Atomic model / Derived calculations / Non-polymer description |
|---|
| Revision 2.0 | Jul 29, 2020 | Group: Advisory / Atomic model ...Advisory / Atomic model / Data collection / Derived calculations / Other / Structure summary
Category: atom_site / chem_comp ...atom_site / chem_comp / entity / pdbx_branch_scheme / pdbx_chem_comp_identifier / pdbx_database_status / pdbx_entity_branch / pdbx_entity_branch_descriptor / pdbx_entity_branch_link / pdbx_entity_branch_list / pdbx_entity_nonpoly / pdbx_nonpoly_scheme / pdbx_struct_assembly_gen / pdbx_struct_conn_angle / pdbx_unobs_or_zero_occ_atoms / struct_asym / struct_conn / struct_site / struct_site_gen
Item: _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ..._atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.type_symbol / _chem_comp.name / _chem_comp.type / _pdbx_database_status.status_code_sf / _pdbx_entity_nonpoly.entity_id / _pdbx_entity_nonpoly.name / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _pdbx_unobs_or_zero_occ_atoms.label_asym_id / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.pdbx_role / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id
Description: Carbohydrate remediation / Provider: repository / Type: Remediation |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components
 Keywords
Keywords HYDROLASE /
HYDROLASE /  NEURAMINIDASE INHIBITOR / NAI /
NEURAMINIDASE INHIBITOR / NAI /  NAIS /
NAIS /  ZANAMIVIR / RESISTANCE / ANTIVIRAL RESISTANCE
ZANAMIVIR / RESISTANCE / ANTIVIRAL RESISTANCE Function and homology information
Function and homology information exo-alpha-sialidase /
exo-alpha-sialidase /  viral budding from plasma membrane / carbohydrate metabolic process / host cell plasma membrane / virion membrane /
viral budding from plasma membrane / carbohydrate metabolic process / host cell plasma membrane / virion membrane /  membrane /
membrane /  metal ion binding
metal ion binding

 INFLUENZA A VIRUS
INFLUENZA A VIRUS X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.9 Å
MOLECULAR REPLACEMENT / Resolution: 1.9 Å  Authors
Authors Citation
Citation Journal: Plos Pathog. / Year: 2012
Journal: Plos Pathog. / Year: 2012 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4b7r.cif.gz
4b7r.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4b7r.ent.gz
pdb4b7r.ent.gz PDB format
PDB format 4b7r.json.gz
4b7r.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/b7/4b7r
https://data.pdbj.org/pub/pdb/validation_reports/b7/4b7r ftp://data.pdbj.org/pub/pdb/validation_reports/b7/4b7r
ftp://data.pdbj.org/pub/pdb/validation_reports/b7/4b7r Links
Links Assembly
Assembly
 Components
Components

 INFLUENZA A VIRUS (A/CALIFORNIA/07/2009(H1N1))
INFLUENZA A VIRUS (A/CALIFORNIA/07/2009(H1N1))
 / Mass: 424.401 Da / Num. of mol.: 1
/ Mass: 424.401 Da / Num. of mol.: 1 N-Acetylglucosamine
N-Acetylglucosamine
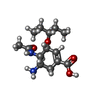





 Oseltamivir
Oseltamivir HEPES
HEPES Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I02 / Wavelength: 0.9795
/ Beamline: I02 / Wavelength: 0.9795  : 0.9795 Å / Relative weight: 1
: 0.9795 Å / Relative weight: 1  Processing
Processing :
:  MOLECULAR REPLACEMENT / Resolution: 1.9→29.818 Å / SU ML: 0.17 / σ(F): 100 / Phase error: 17.4 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 1.9→29.818 Å / SU ML: 0.17 / σ(F): 100 / Phase error: 17.4 / Stereochemistry target values: ML Movie
Movie Controller
Controller
















 PDBj
PDBj





