[English] 日本語
 Yorodumi
Yorodumi- PDB-3zrk: Identification of 2-(4-pyridyl)thienopyridinones as GSK-3beta inh... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3zrk | ||||||
|---|---|---|---|---|---|---|---|
| Title | Identification of 2-(4-pyridyl)thienopyridinones as GSK-3beta inhibitors | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE/PEPTIDE / TRANSFERASE-PEPTIDE COMPLEX /  KINASE KINASE | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of microtubule anchoring at centrosome / negative regulation of glycogen (starch) synthase activity / neuron projection organization / negative regulation of mesenchymal stem cell differentiation / beta-catenin destruction complex disassembly / negative regulation of type B pancreatic cell development / superior temporal gyrus development / positive regulation of protein localization to cilium / negative regulation of glycogen biosynthetic process / negative regulation of dopaminergic neuron differentiation ...regulation of microtubule anchoring at centrosome / negative regulation of glycogen (starch) synthase activity / neuron projection organization / negative regulation of mesenchymal stem cell differentiation / beta-catenin destruction complex disassembly / negative regulation of type B pancreatic cell development / superior temporal gyrus development / positive regulation of protein localization to cilium / negative regulation of glycogen biosynthetic process / negative regulation of dopaminergic neuron differentiation / maintenance of cell polarity / positive regulation of protein localization to centrosome / : / positive regulation of mitochondrial outer membrane permeabilization involved in apoptotic signaling pathway / positive regulation of cilium assembly / negative regulation of protein acetylation / APC truncation mutants have impaired AXIN binding / AXIN missense mutants destabilize the destruction complex / Truncations of AMER1 destabilize the destruction complex / beta-catenin destruction complex /  tau-protein kinase / CRMPs in Sema3A signaling / heart valve development / regulation of microtubule-based process / regulation of protein export from nucleus / Beta-catenin phosphorylation cascade / Signaling by GSK3beta mutants / CTNNB1 S33 mutants aren't phosphorylated / CTNNB1 S37 mutants aren't phosphorylated / CTNNB1 S45 mutants aren't phosphorylated / CTNNB1 T41 mutants aren't phosphorylated / Maturation of nucleoprotein / cellular response to interleukin-3 / Wnt signalosome / negative regulation of protein localization to nucleus / negative regulation of TOR signaling / regulation of long-term synaptic potentiation / Disassembly of the destruction complex and recruitment of AXIN to the membrane / Maturation of nucleoprotein / AKT phosphorylates targets in the cytosol / negative regulation of calcineurin-NFAT signaling cascade / positive regulation of cell-matrix adhesion / regulation of axon extension / dopamine receptor signaling pathway / negative regulation of phosphoprotein phosphatase activity / regulation of dendrite morphogenesis / tau-protein kinase / CRMPs in Sema3A signaling / heart valve development / regulation of microtubule-based process / regulation of protein export from nucleus / Beta-catenin phosphorylation cascade / Signaling by GSK3beta mutants / CTNNB1 S33 mutants aren't phosphorylated / CTNNB1 S37 mutants aren't phosphorylated / CTNNB1 S45 mutants aren't phosphorylated / CTNNB1 T41 mutants aren't phosphorylated / Maturation of nucleoprotein / cellular response to interleukin-3 / Wnt signalosome / negative regulation of protein localization to nucleus / negative regulation of TOR signaling / regulation of long-term synaptic potentiation / Disassembly of the destruction complex and recruitment of AXIN to the membrane / Maturation of nucleoprotein / AKT phosphorylates targets in the cytosol / negative regulation of calcineurin-NFAT signaling cascade / positive regulation of cell-matrix adhesion / regulation of axon extension / dopamine receptor signaling pathway / negative regulation of phosphoprotein phosphatase activity / regulation of dendrite morphogenesis /  regulation of axonogenesis / establishment of cell polarity / regulation of axonogenesis / establishment of cell polarity /  tau-protein kinase activity / glycogen metabolic process / molecular function inhibitor activity / ER overload response / Constitutive Signaling by AKT1 E17K in Cancer / protein kinase A catalytic subunit binding / tau-protein kinase activity / glycogen metabolic process / molecular function inhibitor activity / ER overload response / Constitutive Signaling by AKT1 E17K in Cancer / protein kinase A catalytic subunit binding /  dynactin binding / dynactin binding /  NF-kappaB binding / Regulation of HSF1-mediated heat shock response / canonical Wnt signaling pathway / NF-kappaB binding / Regulation of HSF1-mediated heat shock response / canonical Wnt signaling pathway /  epithelial to mesenchymal transition / negative regulation of osteoblast differentiation / negative regulation of protein-containing complex assembly / positive regulation of autophagy / regulation of microtubule cytoskeleton organization / regulation of cellular response to heat / cellular response to retinoic acid / extrinsic apoptotic signaling pathway / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / extrinsic apoptotic signaling pathway in absence of ligand / presynaptic modulation of chemical synaptic transmission / negative regulation of insulin receptor signaling pathway / epithelial to mesenchymal transition / negative regulation of osteoblast differentiation / negative regulation of protein-containing complex assembly / positive regulation of autophagy / regulation of microtubule cytoskeleton organization / regulation of cellular response to heat / cellular response to retinoic acid / extrinsic apoptotic signaling pathway / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / extrinsic apoptotic signaling pathway in absence of ligand / presynaptic modulation of chemical synaptic transmission / negative regulation of insulin receptor signaling pathway /  excitatory postsynaptic potential / positive regulation of protein export from nucleus / positive regulation of protein ubiquitination / Ubiquitin-dependent degradation of Cyclin D / hippocampus development / positive regulation of cell differentiation / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / peptidyl-threonine phosphorylation / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / positive regulation of protein-containing complex assembly / Degradation of beta-catenin by the destruction complex / tau protein binding / negative regulation of canonical Wnt signaling pathway / B-WICH complex positively regulates rRNA expression / excitatory postsynaptic potential / positive regulation of protein export from nucleus / positive regulation of protein ubiquitination / Ubiquitin-dependent degradation of Cyclin D / hippocampus development / positive regulation of cell differentiation / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / peptidyl-threonine phosphorylation / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / positive regulation of protein-containing complex assembly / Degradation of beta-catenin by the destruction complex / tau protein binding / negative regulation of canonical Wnt signaling pathway / B-WICH complex positively regulates rRNA expression /  regulation of circadian rhythm / regulation of circadian rhythm /  beta-catenin binding / positive regulation of GTPase activity / beta-catenin binding / positive regulation of GTPase activity /  circadian rhythm / positive regulation of protein catabolic process / cellular response to amyloid-beta / Regulation of RUNX2 expression and activity / neuron projection development / positive regulation of neuron apoptotic process / positive regulation of canonical Wnt signaling pathway / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / circadian rhythm / positive regulation of protein catabolic process / cellular response to amyloid-beta / Regulation of RUNX2 expression and activity / neuron projection development / positive regulation of neuron apoptotic process / positive regulation of canonical Wnt signaling pathway / positive regulation of proteasomal ubiquitin-dependent protein catabolic process /  p53 binding / presynapse / positive regulation of protein binding / insulin receptor signaling pathway p53 binding / presynapse / positive regulation of protein binding / insulin receptor signaling pathwaySimilarity search - Function | ||||||
| Biological species |   HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.37 Å FOURIER SYNTHESIS / Resolution: 2.37 Å | ||||||
 Authors Authors | Gentile, G. / Bernasconi, G. / Pozzan, A. / Merlo, G. / Marzorati, P. / Bamborough, P. / Bax, B. / Bridges, A. / Brough, C. / Carter, P. ...Gentile, G. / Bernasconi, G. / Pozzan, A. / Merlo, G. / Marzorati, P. / Bamborough, P. / Bax, B. / Bridges, A. / Brough, C. / Carter, P. / Cutler, G. / Neu, M. / Takada, M. | ||||||
 Citation Citation |  Journal: Bioorg.Med.Chem.Lett. / Year: 2011 Journal: Bioorg.Med.Chem.Lett. / Year: 2011Title: Identification of 2-(4-Pyridyl)Thienopyridinones as Gsk-3Beta Inhibitors. Authors: Gentile, G. / Bernasconi, G. / Pozzan, A. / Merlo, G. / Marzorati, P. / Bamborough, P. / Bax, B. / Bridges, A. / Brough, C. / Carter, P. / Cutler, G. / Neu, M. / Takada, M. | ||||||
| History |
| ||||||
| Remark 700 | SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW ... SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "AA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 6-STRANDED BARREL THIS IS REPRESENTED BY A 7-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. THE SHEETS PRESENTED AS "BA" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 6-STRANDED BARREL THIS IS REPRESENTED BY A 7-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3zrk.cif.gz 3zrk.cif.gz | 169.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3zrk.ent.gz pdb3zrk.ent.gz | 134.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3zrk.json.gz 3zrk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zr/3zrk https://data.pdbj.org/pub/pdb/validation_reports/zr/3zrk ftp://data.pdbj.org/pub/pdb/validation_reports/zr/3zrk ftp://data.pdbj.org/pub/pdb/validation_reports/zr/3zrk | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3zrlC  3zrmC  1gngS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Protein/peptide , 2 types, 4 molecules ABXY
| #1: Protein |  / GSK-3 BETA / GSK-3 BETAMass: 42036.117 Da / Num. of mol.: 2 / Fragment: RESIDUES 23-393 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   HOMO SAPIENS (human) / Cell line (production host): SF9 / Production host: HOMO SAPIENS (human) / Cell line (production host): SF9 / Production host:   SPODOPTERA FRUGIPERDA (fall armyworm) / References: UniProt: P49841, SPODOPTERA FRUGIPERDA (fall armyworm) / References: UniProt: P49841,  tau-protein kinase tau-protein kinase#2: Protein/peptide | Mass: 3772.434 Da / Num. of mol.: 2 / Fragment: RESIDUES 197-226 Source method: isolated from a genetically manipulated source Details: FRATTIDE SEQUENCE CO-EXPRESSED IN BACULOVIRUS (DUAL EXPRESSION). Source: (gene. exp.)   HOMO SAPIENS (human) / Cell line (production host): SF9 / Production host: HOMO SAPIENS (human) / Cell line (production host): SF9 / Production host:   SPODOPTERA FRUGIPERDA (fall armyworm) / References: UniProt: Q92837 SPODOPTERA FRUGIPERDA (fall armyworm) / References: UniProt: Q92837 |
|---|
-Non-polymers , 4 types, 220 molecules 

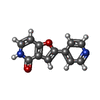




| #3: Chemical | ChemComp-SO4 /  Sulfate Sulfate#4: Chemical |  Glycerol Glycerol#5: Chemical | #6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.21 Å3/Da / Density % sol: 44.27 % / Description: NONE |
|---|---|
Crystal grow | Details: 0.2M AMMONIUM SULFATE, 0.1M BISTRIS PH6.5, 30% PEG 3350, 10% GLYCEROL |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 1.07225 / Beamline: ID23-1 / Wavelength: 1.07225 |
| Detector | Type: ADSC CCD / Detector: CCD |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.07225 Å / Relative weight: 1 : 1.07225 Å / Relative weight: 1 |
| Reflection | Resolution: 2.37→40 Å / Num. obs: 36362 / % possible obs: 99.9 % / Observed criterion σ(I): 0 / Redundancy: 7 % / Rmerge(I) obs: 0.07 / Net I/σ(I): 28.2 |
| Reflection shell | Resolution: 2.37→2.41 Å / Redundancy: 6.5 % / Rmerge(I) obs: 0.45 / Mean I/σ(I) obs: 4.3 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: PDB ENTRY 1GNG Resolution: 2.37→20 Å / Cor.coef. Fo:Fc: 0.948 / Cor.coef. Fo:Fc free: 0.927 / SU B: 7.152 / SU ML: 0.173 / Cross valid method: THROUGHOUT / ESU R: 0.401 / ESU R Free: 0.251 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 45.527 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.37→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller
































 PDBj
PDBj








