[English] 日本語
 Yorodumi
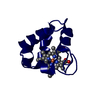
Yorodumi- PDB-3zow: Crystal Structure of Wild Type Nitrosomonas europaea Cytochrome c552 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3zow | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Wild Type Nitrosomonas europaea Cytochrome c552 | ||||||
 Components Components | CYTOCHROME C-552 | ||||||
 Keywords Keywords |  ELECTRON TRANSPORT / ELECTRON TRANSPORT /  HEMEPROTEIN HEMEPROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology information periplasmic space / periplasmic space /  electron transfer activity / iron ion binding / electron transfer activity / iron ion binding /  heme binding heme bindingSimilarity search - Function | ||||||
| Biological species |   NITROSOMONAS EUROPAEA (bacteria) NITROSOMONAS EUROPAEA (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.35 Å MOLECULAR REPLACEMENT / Resolution: 2.35 Å | ||||||
 Authors Authors | Hersleth, H.-P. / Can, M. / Krucinska, J. / Zoppellaro, G. / Andersen, N.H. / Karlsen, S. / Wedekind, J.E. / Andersson, K.K. / Bren, K.L. | ||||||
 Citation Citation |  Journal: Chembiochem / Year: 2013 Journal: Chembiochem / Year: 2013Title: Structural Characterization of Nitrosomonas Europaea Cytochrome C-552 Variants with Marked Differences in Electronic Structure. Authors: Can, M. / Krucinska, J. / Zoppellaro, G. / Andersen, N.H. / Wedekind, J.E. / Hersleth, H.-P. / Andersson, K.K. / Bren, K.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3zow.cif.gz 3zow.cif.gz | 549.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3zow.ent.gz pdb3zow.ent.gz | 465.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3zow.json.gz 3zow.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zo/3zow https://data.pdbj.org/pub/pdb/validation_reports/zo/3zow ftp://data.pdbj.org/pub/pdb/validation_reports/zo/3zow ftp://data.pdbj.org/pub/pdb/validation_reports/zo/3zow | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3zoxSC  3zoyC  4jcgC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
- Components
Components
| #1: Protein | Mass: 8491.702 Da / Num. of mol.: 18 / Source method: isolated from a natural source / Source: (natural)   NITROSOMONAS EUROPAEA (bacteria) NITROSOMONAS EUROPAEA (bacteria)References: UniProt: P95339,  nitrite reductase (cytochrome; ammonia-forming) nitrite reductase (cytochrome; ammonia-forming)#2: Chemical | ChemComp-HEC /  Heme C Heme C#3: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.57 Å3/Da / Density % sol: 51.9 % / Description: NONE |
|---|---|
Crystal grow | pH: 8 / Details: 3 M AMMONIUM SULFATE, 10 MM TRIS-HCL PH 8 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: X11 / Wavelength: 0.8496 / Beamline: X11 / Wavelength: 0.8496 |
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Oct 24, 2001 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.8496 Å / Relative weight: 1 : 0.8496 Å / Relative weight: 1 |
| Reflection | Resolution: 2.35→38.8 Å / Num. obs: 68939 / % possible obs: 97.1 % / Observed criterion σ(I): 6 / Redundancy: 3.3 % / Rmerge(I) obs: 0.06 / Net I/σ(I): 13.6 |
| Reflection shell | Resolution: 2.35→2.48 Å / Redundancy: 2.8 % / Rmerge(I) obs: 0.3 / Mean I/σ(I) obs: 3.2 / % possible all: 89.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3ZOX Resolution: 2.35→35.22 Å / Cor.coef. Fo:Fc: 0.932 / Cor.coef. Fo:Fc free: 0.891 / SU B: 13.213 / SU ML: 0.188 / Cross valid method: THROUGHOUT / ESU R: 0.382 / ESU R Free: 0.263 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT. IT IS COMMON IN PUBLICATIONS ETC. TO START THE NUMBERING OF THE CYTOCHROME C552 ...Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT. IT IS COMMON IN PUBLICATIONS ETC. TO START THE NUMBERING OF THE CYTOCHROME C552 NITROSOMONAS EUROPAEA WITH THE FIRST RESIDUE IN THE SEQUENCE BEING NUMBERED AS RESIDUE 3. SINGLE-CRYSTAL UV-VIS SPECTRA HAVE BEEN RECORDED BEFORE AND AFTER EXPOSURE TO X-RAYS SHOWING THE RADIATION-INFLUENCE OF THE FERRIC CYTOCHROME C552 CRYSTALS SEE JRNL.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 37.493 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.35→35.22 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj