+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3s1z | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of acetamide bound Xanthomonas campestri OleA | ||||||
 Components Components | 3-oxoacyl-[ACP] synthase III | ||||||
 Keywords Keywords |  TRANSFERASE / Non-decarboxylative Claisen Condensation TRANSFERASE / Non-decarboxylative Claisen Condensation | ||||||
| Function / homology |  Function and homology information Function and homology informationacyl-CoA:acyl-CoA alkyltransferase / secondary metabolite biosynthetic process / 3-oxoacyl-[acyl-carrier-protein] synthase activity / fatty acid biosynthetic process /  metal ion binding / metal ion binding /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Xanthomonas campestris pv. campestris (bacteria) Xanthomonas campestris pv. campestris (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.0547 Å FOURIER SYNTHESIS / Resolution: 2.0547 Å | ||||||
 Authors Authors | Goblirsch, B.R. / Wilmot, C.M. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2012 Journal: Biochemistry / Year: 2012Title: Crystal Structures of Xanthomonas campestris OleA Reveal Features That Promote Head-to-Head Condensation of Two Long-Chain Fatty Acids. Authors: Goblirsch, B.R. / Frias, J.A. / Wackett, L.P. / Wilmot, C.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3s1z.cif.gz 3s1z.cif.gz | 275.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3s1z.ent.gz pdb3s1z.ent.gz | 222.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3s1z.json.gz 3s1z.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/s1/3s1z https://data.pdbj.org/pub/pdb/validation_reports/s1/3s1z ftp://data.pdbj.org/pub/pdb/validation_reports/s1/3s1z ftp://data.pdbj.org/pub/pdb/validation_reports/s1/3s1z | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3rowSC  3s20C  3s21C  3s23C S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
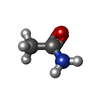
| #1: Protein | Mass: 37306.805 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Xanthomonas campestris pv. campestris (bacteria) Xanthomonas campestris pv. campestris (bacteria)Gene: fabH, XCC0212 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q8PDX2 Escherichia coli (E. coli) / References: UniProt: Q8PDX2#2: Chemical |  Acetamide Acetamide#3: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.4 Å3/Da / Density % sol: 48.68 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 4.2 Details: 18% PEG 8000, 80 mM potassium phosphate dibasic, 100 mM sodium citrate pH 4.2, VAPOR DIFFUSION, HANGING DROP, temperature 298.0K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 23-ID-B / Wavelength: 1.03 Å / Beamline: 23-ID-B / Wavelength: 1.03 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Feb 19, 2011 |
| Radiation | Monochromator: Si 111 CHANNEL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.03 Å / Relative weight: 1 : 1.03 Å / Relative weight: 1 |
| Reflection | Resolution: 2.05→50 Å / Num. all: 45354 / Num. obs: 45354 / % possible obs: 100 % / Observed criterion σ(F): -3 / Observed criterion σ(I): 0 / Redundancy: 8.1 % / Biso Wilson estimate: 25.41 Å2 / Rmerge(I) obs: 0.079 / Net I/σ(I): 33.6 |
| Reflection shell | Resolution: 2.05→2.09 Å / Redundancy: 7.6 % / Rmerge(I) obs: 0.489 / Mean I/σ(I) obs: 7.6 / % possible all: 99.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: PDB ENTRY 3ROW Resolution: 2.0547→30.432 Å / SU ML: 0.23 / σ(F): 0 / Phase error: 17.51 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.95 Å / VDW probe radii: 1.2 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 43.395 Å2 / ksol: 0.359 e/Å3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.0547→30.432 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -12.1748 Å / Origin y: 15.1294 Å / Origin z: -14.2634 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: (chain 'A') or (chain 'B') |
 Movie
Movie Controller
Controller













 PDBj
PDBj