[English] 日本語
 Yorodumi
Yorodumi- PDB-3qrm: HIV-1 protease (mutant Q7K L33I L63I) in complex with a three-arm... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3qrm | ||||||
|---|---|---|---|---|---|---|---|
| Title | HIV-1 protease (mutant Q7K L33I L63I) in complex with a three-armed pyrrolidine-based inhibitor | ||||||
 Components Components | Protease | ||||||
 Keywords Keywords | HYDROLASE/HYDROLASE INHIBITOR /  Aspartyl Protease / HYDROLASE-HYDROLASE INHIBITOR complex Aspartyl Protease / HYDROLASE-HYDROLASE INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationRNA stem-loop binding /  host cell / host cell /  HIV-1 retropepsin / HIV-1 retropepsin /  retroviral ribonuclease H / retroviral ribonuclease H /  exoribonuclease H / exoribonuclease H /  exoribonuclease H activity / host multivesicular body / DNA integration / exoribonuclease H activity / host multivesicular body / DNA integration /  RNA-directed DNA polymerase / viral genome integration into host DNA ...RNA stem-loop binding / RNA-directed DNA polymerase / viral genome integration into host DNA ...RNA stem-loop binding /  host cell / host cell /  HIV-1 retropepsin / HIV-1 retropepsin /  retroviral ribonuclease H / retroviral ribonuclease H /  exoribonuclease H / exoribonuclease H /  exoribonuclease H activity / host multivesicular body / DNA integration / exoribonuclease H activity / host multivesicular body / DNA integration /  RNA-directed DNA polymerase / viral genome integration into host DNA / viral penetration into host nucleus / establishment of integrated proviral latency / RNA-directed DNA polymerase / viral genome integration into host DNA / viral penetration into host nucleus / establishment of integrated proviral latency /  RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity /  Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / symbiont-mediated suppression of host gene expression / viral nucleocapsid / DNA recombination / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / symbiont-mediated suppression of host gene expression / viral nucleocapsid / DNA recombination /  Hydrolases; Acting on ester bonds / Hydrolases; Acting on ester bonds /  DNA-directed DNA polymerase / aspartic-type endopeptidase activity / DNA-directed DNA polymerase / aspartic-type endopeptidase activity /  DNA-directed DNA polymerase activity / symbiont entry into host cell / DNA-directed DNA polymerase activity / symbiont entry into host cell /  lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity /  proteolysis / proteolysis /  DNA binding / zinc ion binding / DNA binding / zinc ion binding /  membrane membraneSimilarity search - Function | ||||||
| Biological species |    Human immunodeficiency virus type 1 Human immunodeficiency virus type 1 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.731 Å MOLECULAR REPLACEMENT / Resolution: 1.731 Å | ||||||
 Authors Authors | Lindemann, I. / Heine, A. / Klebe, G. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Design of a series of novel three-armed pyrrolidine-based inhibitors for HIV-1 protease Authors: Lindemann, I. / Klee, N. / Heine, A. / Diederich, W.E. / Klebe, G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3qrm.cif.gz 3qrm.cif.gz | 60.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3qrm.ent.gz pdb3qrm.ent.gz | 42.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3qrm.json.gz 3qrm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qr/3qrm https://data.pdbj.org/pub/pdb/validation_reports/qr/3qrm ftp://data.pdbj.org/pub/pdb/validation_reports/qr/3qrm ftp://data.pdbj.org/pub/pdb/validation_reports/qr/3qrm | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3qbfC  3qpjC  3qroC  3qrsC  2pqzS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 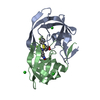
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
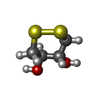
| #1: Protein |  / PR / Retropepsin / PR / RetropepsinMass: 10804.808 Da / Num. of mol.: 2 / Mutation: Q7K L33I L63I Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus type 1 (BRU ISOLATE) Human immunodeficiency virus type 1 (BRU ISOLATE)Plasmid: pET24a / Production host:   Escherichia coli (E. coli) / References: UniProt: P03367, Escherichia coli (E. coli) / References: UniProt: P03367,  HIV-1 retropepsin HIV-1 retropepsin#2: Chemical | ChemComp-NK7 / | #3: Chemical |  Chloride Chloride#4: Chemical | ChemComp-DTD / | #5: Water | ChemComp-HOH / |  Water WaterNonpolymer details | NEXT TO THE DTD MOLECULE IN THE BINDING POCKET WE OBSERVED ADDITIONAL DENSITY (5.00 SIGMA LEVEL) ...NEXT TO THE DTD MOLECULE IN THE BINDING POCKET WE OBSERVED ADDITIONAL | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.68 Å3/Da / Density % sol: 54.13 % |
|---|---|
Crystal grow | Temperature: 289 K / Method: vapor diffusion, sitting drop / pH: 5.5 Details: 700mM NaCl, 100mM Na citrate, 100mM DTT, 3mM NaN3, pH 5.5, VAPOR DIFFUSION, SITTING DROP, temperature 289K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  BESSY BESSY  / Beamline: 14.2 / Wavelength: 0.91841 Å / Beamline: 14.2 / Wavelength: 0.91841 Å |
| Detector | Type: RAYONIX MX-225 / Detector: CCD / Date: Mar 26, 2010 / Details: mirrors |
| Radiation | Monochromator: Double Crystal Monochromator KMC-2 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.91841 Å / Relative weight: 1 : 0.91841 Å / Relative weight: 1 |
| Reflection | Resolution: 1.73→30 Å / Num. all: 24130 / Num. obs: 24130 / % possible obs: 100 % / Redundancy: 3.6 % / Biso Wilson estimate: 13.59 Å2 / Rsym value: 0.044 / Net I/σ(I): 28.8 |
| Reflection shell | Resolution: 1.73→1.76 Å / Redundancy: 3.6 % / Mean I/σ(I) obs: 6 / Num. unique all: 1150 / Rsym value: 0.19 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2PQZ Resolution: 1.731→25.701 Å / Occupancy max: 1 / Occupancy min: 0.23 / SU ML: 0.2 / σ(F): 0 / Phase error: 18.71 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.72 Å / VDW probe radii: 1 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 39.573 Å2 / ksol: 0.374 e/Å3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 61.18 Å2 / Biso mean: 16.0412 Å2 / Biso min: 3.69 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.731→25.701 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 10
|
 Movie
Movie Controller
Controller



















 PDBj
PDBj