[English] 日本語
 Yorodumi
Yorodumi- PDB-3ll5: Crystal structure of T. acidophilum isopentenyl phosphate kinase ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3ll5 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of T. acidophilum isopentenyl phosphate kinase product complex | ||||||
 Components Components | Gamma-glutamyl kinase related protein | ||||||
 Keywords Keywords |  TRANSFERASE / alternate mevalonate pathway / isopentenyl phsophate kinase / alpha-beta-alpha sandwich fold / product complex / TRANSFERASE / alternate mevalonate pathway / isopentenyl phsophate kinase / alpha-beta-alpha sandwich fold / product complex /  Kinase Kinase | ||||||
| Function / homology |  Function and homology information Function and homology information isopentenyl phosphate kinase / isopentenyl phosphate kinase /  isopentenyl phosphate kinase activity / isoprenoid biosynthetic process / isopentenyl phosphate kinase activity / isoprenoid biosynthetic process /  kinase activity / kinase activity /  phosphorylation / phosphorylation /  ATP binding ATP bindingSimilarity search - Function | ||||||
| Biological species |    Thermoplasma acidophilum (acidophilic) Thermoplasma acidophilum (acidophilic) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.987 Å MOLECULAR REPLACEMENT / Resolution: 1.987 Å | ||||||
 Authors Authors | Mabanglo, M.F. / Hill, C.P. | ||||||
 Citation Citation |  Journal: Acs Chem.Biol. / Year: 2010 Journal: Acs Chem.Biol. / Year: 2010Title: X-ray structures of isopentenyl phosphate kinase. Authors: Mabanglo, M.F. / Schubert, H.L. / Chen, M. / Hill, C.P. / Poulter, C.D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3ll5.cif.gz 3ll5.cif.gz | 203.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3ll5.ent.gz pdb3ll5.ent.gz | 164.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3ll5.json.gz 3ll5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ll/3ll5 https://data.pdbj.org/pub/pdb/validation_reports/ll/3ll5 ftp://data.pdbj.org/pub/pdb/validation_reports/ll/3ll5 ftp://data.pdbj.org/pub/pdb/validation_reports/ll/3ll5 | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 28107.473 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)    Thermoplasma acidophilum (acidophilic) / Strain: DSM1728 / Gene: Ta0103 / Plasmid: pET151/D-TOPO / Production host: Thermoplasma acidophilum (acidophilic) / Strain: DSM1728 / Gene: Ta0103 / Plasmid: pET151/D-TOPO / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3) Codon Plus RIL / References: UniProt: Q9HLX1 Escherichia coli (E. coli) / Strain (production host): BL21(DE3) Codon Plus RIL / References: UniProt: Q9HLX1 |
|---|
-Non-polymers , 5 types, 248 molecules 
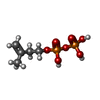

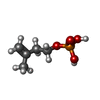





| #2: Chemical |  Adenosine diphosphate Adenosine diphosphate#3: Chemical |  Isopentenyl pyrophosphate Isopentenyl pyrophosphate#4: Chemical |  Adenosine triphosphate Adenosine triphosphate#5: Chemical | #6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.21 Å3/Da / Density % sol: 44.28 % |
|---|---|
Crystal grow | Temperature: 274 K / Method: vapor diffusion, sitting drop / pH: 6 Details: 0.1 M PCB buffer (2:1:2 molar ratio of sodium propionate, sodium cacodylate, Bis-Tris propane, pH 6.0 containing 25% PEG1500, VAPOR DIFFUSION, SITTING DROP, temperature 274K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL11-1 / Wavelength: 0.97887 Å / Beamline: BL11-1 / Wavelength: 0.97887 Å |
| Detector | Type: MARMOSAIC 325 mm CCD / Detector: CCD / Date: Feb 27, 2009 Details: Flat mirror (vertical focusing); single crystal Si(111) bent monochromator (horizontal focusing) |
| Radiation | Monochromator: Side scattering bent cube-root I-beam single crystal; asymmetric cut 4.965 degs Protocol: MAD / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.97887 Å / Relative weight: 1 : 0.97887 Å / Relative weight: 1 |
| Reflection | Resolution: 1.99→50 Å / Num. obs: 65826 / Redundancy: 1.8 % / Rsym value: 0.062 / Net I/σ(I): 9.48 |
| Reflection shell | Resolution: 1.99→2.06 Å / Redundancy: 1.7 % / Mean I/σ(I) obs: 3.3 / Rsym value: 0.062 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: T. acidophilum isopentenyl phosphate kinase solved by single wavelength anomalous diffraction Resolution: 1.987→38.292 Å / SU ML: 0.26 / σ(F): 1.34 / Phase error: 23.97 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 29.941 Å2 / ksol: 0.425 e/Å3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.987→38.292 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj



