[English] 日本語
 Yorodumi
Yorodumi- PDB-3ldb: Structure of JMJD6 complexd with ALPHA-KETOGLUTARATE and Fab Fragment. -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3ldb | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of JMJD6 complexd with ALPHA-KETOGLUTARATE and Fab Fragment. | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  IMMUNE SYSTEM / JMJ6D / IMMUNE SYSTEM / JMJ6D /  ALPHA-KETOGLUTARATE / Fab Fragments ALPHA-KETOGLUTARATE / Fab Fragments | ||||||
| Function / homology |  Function and homology information Function and homology informationpeptidyl-lysine hydroxylation to 5-hydroxy-L-lysine / histone H3R2 demethylase activity / peptidyl-lysine 5-dioxygenase activity / histone H4R3 demethylase activity / protein demethylase activity / recognition of apoptotic cell / negative regulation of protein homooligomerization / oxidative RNA demethylation / oxidative RNA demethylase activity / non-membrane-bounded organelle assembly ...peptidyl-lysine hydroxylation to 5-hydroxy-L-lysine / histone H3R2 demethylase activity / peptidyl-lysine 5-dioxygenase activity / histone H4R3 demethylase activity / protein demethylase activity / recognition of apoptotic cell / negative regulation of protein homooligomerization / oxidative RNA demethylation / oxidative RNA demethylase activity / non-membrane-bounded organelle assembly /  Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With 2-oxoglutarate as one donor, and incorporation of one atom of oxygen into each donor / Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With 2-oxoglutarate as one donor, and incorporation of one atom of oxygen into each donor /  macrophage activation / transcription regulator activator activity / macrophage activation / transcription regulator activator activity /  regulation of mRNA splicing, via spliceosome / regulation of mRNA splicing, via spliceosome /  sprouting angiogenesis / erythrocyte development / sprouting angiogenesis / erythrocyte development /  Protein hydroxylation / P-TEFb complex binding / histone demethylase activity / Protein hydroxylation / P-TEFb complex binding / histone demethylase activity /  phagocytosis / phagocytosis /  RNA splicing / RNA splicing /  kidney development / lung development / protein homooligomerization / HDMs demethylate histones / kidney development / lung development / protein homooligomerization / HDMs demethylate histones /  mRNA processing / retina development in camera-type eye / mRNA processing / retina development in camera-type eye /  signaling receptor activity / T cell differentiation in thymus / signaling receptor activity / T cell differentiation in thymus /  heart development / heart development /  single-stranded RNA binding / cell surface receptor signaling pathway / single-stranded RNA binding / cell surface receptor signaling pathway /  chromatin remodeling / iron ion binding / chromatin remodeling / iron ion binding /  ribonucleoprotein complex / ribonucleoprotein complex /  nucleolus / positive regulation of DNA-templated transcription / positive regulation of transcription by RNA polymerase II / nucleolus / positive regulation of DNA-templated transcription / positive regulation of transcription by RNA polymerase II /  RNA binding / RNA binding /  nucleoplasm / identical protein binding / nucleoplasm / identical protein binding /  nucleus / nucleus /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human)  Cricetulus migratorius (Armenian hamster) Cricetulus migratorius (Armenian hamster) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.7 Å MOLECULAR REPLACEMENT / Resolution: 2.7 Å | ||||||
 Authors Authors | Hong, X. / Zang, J. / White, J. / Kappler, J.W. / Wang, C. / Zhang, G. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2010 Journal: Proc.Natl.Acad.Sci.USA / Year: 2010Title: Interaction of JMJD6 with single-stranded RNA. Authors: Hong, X. / Zang, J. / White, J. / Wang, C. / Pan, C.H. / Zhao, R. / Murphy, R.C. / Dai, S. / Henson, P. / Kappler, J.W. / Hagman, J. / Zhang, G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3ldb.cif.gz 3ldb.cif.gz | 169.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3ldb.ent.gz pdb3ldb.ent.gz | 132.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3ldb.json.gz 3ldb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ld/3ldb https://data.pdbj.org/pub/pdb/validation_reports/ld/3ldb ftp://data.pdbj.org/pub/pdb/validation_reports/ld/3ldb ftp://data.pdbj.org/pub/pdb/validation_reports/ld/3ldb | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3ld8SC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 39437.746 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: JMJD6, JMJD6 (1-403), KIAA0585, PTDSR / Plasmid: pET23b / Production host: Homo sapiens (human) / Gene: JMJD6, JMJD6 (1-403), KIAA0585, PTDSR / Plasmid: pET23b / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21 Escherichia coli (E. coli) / Strain (production host): BL21References: UniProt: Q6NYC1,  Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With 2-oxoglutarate as one donor, and incorporation of one atom of oxygen into each donor Oxidoreductases; Acting on paired donors, with incorporation or reduction of molecular oxygen; With 2-oxoglutarate as one donor, and incorporation of one atom of oxygen into each donor |
|---|
-Antibody , 2 types, 2 molecules BC
| #2: Antibody | Mass: 24100.004 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Cricetulus migratorius (Armenian hamster) Cricetulus migratorius (Armenian hamster)Cell: hybridoma / Production host:   Cricetulus migratorius (Armenian hamster) Cricetulus migratorius (Armenian hamster) |
|---|---|
| #3: Antibody | Mass: 24007.662 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Cricetulus migratorius (Armenian hamster) Cricetulus migratorius (Armenian hamster)Cell: hybridoma / Production host:   Cricetulus migratorius (Armenian hamster) Cricetulus migratorius (Armenian hamster) |
-Non-polymers , 5 types, 28 molecules 

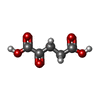






| #4: Chemical | ChemComp-GOL /  Glycerol Glycerol#5: Chemical | ChemComp-SO4 /  Sulfate Sulfate#6: Chemical | ChemComp-AKG / |  Α-Ketoglutaric acid Α-Ketoglutaric acid#7: Chemical | ChemComp-FE / |  Iron Iron#8: Chemical |  Mercury (element) Mercury (element) |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 3 X-RAY DIFFRACTION / Number of used crystals: 3 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 5.02 Å3/Da / Density % sol: 75.5 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 5.6 Details: 0.1 M Bis(2-hydroxyethyl)-amino-tris(hydroxymethyl)-methane, 2.1 M Ammonium Sulphate, pH 5.6, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source |
| |||||||||||||||
| Detector |
| |||||||||||||||
| Radiation |
| |||||||||||||||
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 | |||||||||||||||
| Reflection | Resolution: 2.61→99 Å / Num. all: 54702 / Num. obs: 48696 / % possible obs: 89 % / Biso Wilson estimate: 66.1 Å2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entries 3LD8, JMJD6, and Fab Fragment Resolution: 2.7→47.28 Å / Rfactor Rfree error: 0.006 / Data cutoff high absF: 3487182.74 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 / Details: BULK SOLVENT MODEL USED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 58.7914 Å2 / ksol: 0.35 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 83.5 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.7→47.28 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | NCS model details: NONE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.7→2.87 Å / Rfactor Rfree error: 0.039 / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj























