[English] 日本語
 Yorodumi
Yorodumi- PDB-3jcu: Cryo-EM structure of spinach PSII-LHCII supercomplex at 3.2 Angst... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3jcu | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of spinach PSII-LHCII supercomplex at 3.2 Angstrom resolution | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  MEMBRANE PROTEIN MEMBRANE PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology information chloroplast photosystem II / chloroplast photosystem II /  plastoglobule / plastoglobule /  photosynthesis, light harvesting in photosystem I / photosynthesis, light harvesting in photosystem I /  : / : /  photosynthesis, light harvesting / photosynthesis, light harvesting /  photosystem II assembly / photosystem II assembly /  photosystem II stabilization / oxygen evolving activity / photosystem II stabilization / oxygen evolving activity /  photosystem II oxygen evolving complex / photosystem II oxygen evolving complex /  photosystem II ... photosystem II ... chloroplast photosystem II / chloroplast photosystem II /  plastoglobule / plastoglobule /  photosynthesis, light harvesting in photosystem I / photosynthesis, light harvesting in photosystem I /  : / : /  photosynthesis, light harvesting / photosynthesis, light harvesting /  photosystem II assembly / photosystem II assembly /  photosystem II stabilization / oxygen evolving activity / photosystem II stabilization / oxygen evolving activity /  photosystem II oxygen evolving complex / photosystem II oxygen evolving complex /  photosystem II / photosystem II /  photosystem II reaction center / photosystem II reaction center /  chloroplast envelope / chloroplast envelope /  : / : /  ion binding / photosynthetic electron transport chain / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor / ion binding / photosynthetic electron transport chain / oxidoreductase activity, acting on diphenols and related substances as donors, oxygen as acceptor /  photosystem I / response to herbicide / photosystem I / response to herbicide /  photosystem II / photosystem II /  extrinsic component of membrane / photosynthetic electron transport in photosystem II / extrinsic component of membrane / photosynthetic electron transport in photosystem II /  chlorophyll binding / chloroplast thylakoid membrane / chlorophyll binding / chloroplast thylakoid membrane /  photosynthesis, light reaction / electron transporter, transferring electrons within the cyclic electron transport pathway of photosynthesis activity / photosynthesis, light reaction / electron transporter, transferring electrons within the cyclic electron transport pathway of photosynthesis activity /  phosphate ion binding / response to light stimulus / phosphate ion binding / response to light stimulus /  photosynthesis / photosynthesis /  phosphoprotein binding / phosphoprotein binding /  electron transfer activity / membrane => GO:0016020 / protein stabilization / iron ion binding / electron transfer activity / membrane => GO:0016020 / protein stabilization / iron ion binding /  calcium ion binding / calcium ion binding /  heme binding / heme binding /  metal ion binding metal ion bindingSimilarity search - Function | ||||||
| Biological species |   Spinacia oleracea (spinach) Spinacia oleracea (spinach) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.2 Å cryo EM / Resolution: 3.2 Å | ||||||
 Authors Authors | Wei, X.P. / Zhang, X.Z. / Su, X.D. / Cao, P. / Liu, X.Y. / Li, M. / Chang, W.R. / Liu, Z.F. | ||||||
 Citation Citation |  Journal: Nature / Year: 2016 Journal: Nature / Year: 2016Title: Structure of spinach photosystem II-LHCII supercomplex at 3.2 Å resolution. Authors: Xuepeng Wei / Xiaodong Su / Peng Cao / Xiuying Liu / Wenrui Chang / Mei Li / Xinzheng Zhang / Zhenfeng Liu /  Abstract: During photosynthesis, the plant photosystem II core complex receives excitation energy from the peripheral light-harvesting complex II (LHCII). The pathways along which excitation energy is ...During photosynthesis, the plant photosystem II core complex receives excitation energy from the peripheral light-harvesting complex II (LHCII). The pathways along which excitation energy is transferred between them, and their assembly mechanisms, remain to be deciphered through high-resolution structural studies. Here we report the structure of a 1.1-megadalton spinach photosystem II-LHCII supercomplex solved at 3.2 Å resolution through single-particle cryo-electron microscopy. The structure reveals a homodimeric supramolecular system in which each monomer contains 25 protein subunits, 105 chlorophylls, 28 carotenoids and other cofactors. Three extrinsic subunits (PsbO, PsbP and PsbQ), which are essential for optimal oxygen-evolving activity of photosystem II, form a triangular crown that shields the Mn4CaO5-binding domains of CP43 and D1. One major trimeric and two minor monomeric LHCIIs associate with each core-complex monomer, and the antenna-core interactions are reinforced by three small intrinsic subunits (PsbW, PsbH and PsbZ). By analysing the closely connected interfacial chlorophylls, we have obtained detailed insights into the energy-transfer pathways between the antenna and core complexes. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3jcu.cif.gz 3jcu.cif.gz | 1.9 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3jcu.ent.gz pdb3jcu.ent.gz | 1.7 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3jcu.json.gz 3jcu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jc/3jcu https://data.pdbj.org/pub/pdb/validation_reports/jc/3jcu ftp://data.pdbj.org/pub/pdb/validation_reports/jc/3jcu ftp://data.pdbj.org/pub/pdb/validation_reports/jc/3jcu | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6617MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Photosystem II ... , 13 types, 26 molecules AaBbCcDdHhJjKkMmTtUuWwXxZz
| #1: Protein |  Mass: 38134.430 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P69560 Spinacia oleracea (spinach) / References: UniProt: P69560#2: Protein |  Mass: 56207.852 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P04160 Spinacia oleracea (spinach) / References: UniProt: P04160#3: Protein |  Mass: 51880.559 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P06003 Spinacia oleracea (spinach) / References: UniProt: P06003#4: Protein |  Mass: 39535.172 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P06005 Spinacia oleracea (spinach) / References: UniProt: P06005#8: Protein |  Mass: 7735.004 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P05146 Spinacia oleracea (spinach) / References: UniProt: P05146#10: Protein/peptide |  Mass: 4117.832 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: Q9M3L2 Spinacia oleracea (spinach) / References: UniProt: Q9M3L2#11: Protein |  Mass: 6751.023 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P12163 Spinacia oleracea (spinach) / References: UniProt: P12163#13: Protein/peptide |  Mass: 3783.538 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P62112 Spinacia oleracea (spinach) / References: UniProt: P62112#19: Protein/peptide |  Mass: 3825.642 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P61840 Spinacia oleracea (spinach) / References: UniProt: P61840#20: Protein |  Mass: 10676.572 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: A0A0K9RHP1 Spinacia oleracea (spinach) / References: UniProt: A0A0K9RHP1#21: Protein |  Mass: 14186.097 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: Q41387 Spinacia oleracea (spinach) / References: UniProt: Q41387#22: Protein |  Mass: 11958.141 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: A0A0K9RTU3, UniProt: A0A0K9RHP1*PLUS Spinacia oleracea (spinach) / References: UniProt: A0A0K9RTU3, UniProt: A0A0K9RHP1*PLUS#23: Protein |  Mass: 6541.715 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: Q9M3M6 Spinacia oleracea (spinach) / References: UniProt: Q9M3M6 |
|---|
-Cytochrome b559 subunit ... , 2 types, 4 molecules EeFf
| #5: Protein |  Mass: 9393.501 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P69383 Spinacia oleracea (spinach) / References: UniProt: P69383#6: Protein/peptide |  Mass: 4502.351 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P60128 Spinacia oleracea (spinach) / References: UniProt: P60128 |
|---|
-Protein Photosystem II reaction center protein ... , 2 types, 4 molecules IiLl
| #9: Protein/peptide |  Mass: 4170.891 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P62103 Spinacia oleracea (spinach) / References: UniProt: P62103#12: Protein/peptide |  Mass: 4498.100 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P60150 Spinacia oleracea (spinach) / References: UniProt: P60150 |
|---|
-Oxygen-evolving enhancer protein ... , 3 types, 6 molecules OoPpQq
| #14: Protein | Mass: 35207.383 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P12359 Spinacia oleracea (spinach) / References: UniProt: P12359#15: Protein | Mass: 28495.918 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P12302 Spinacia oleracea (spinach) / References: UniProt: P12302#16: Protein | Mass: 24884.426 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P12301 Spinacia oleracea (spinach) / References: UniProt: P12301 |
|---|
-Chlorophyll A-B Binding protein ... , 2 types, 4 molecules RrSs
| #17: Protein | Mass: 26735.303 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: F2Z293, UniProt: A0A0K9QLX6*PLUS Spinacia oleracea (spinach) / References: UniProt: F2Z293, UniProt: A0A0K9QLX6*PLUS#18: Protein | Mass: 31341.840 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: A0A0K9QUQ7 Spinacia oleracea (spinach) / References: UniProt: A0A0K9QUQ7 |
|---|
-Protein / Sugars , 2 types, 14 molecules GNYgny

| #32: Sugar | ChemComp-DGD / #7: Protein | Mass: 28449.391 Da / Num. of mol.: 6 / Source method: isolated from a natural source / Source: (natural)   Spinacia oleracea (spinach) / References: UniProt: P12333 Spinacia oleracea (spinach) / References: UniProt: P12333 |
|---|
-Non-polymers , 16 types, 314 molecules 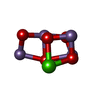





























| #24: Chemical | | #25: Chemical | #26: Chemical | ChemComp-CL /  Chloride Chloride#27: Chemical | ChemComp-CLA /  Chlorophyll a Chlorophyll a#28: Chemical | ChemComp-PHO /  Pheophytin Pheophytin#29: Chemical | ChemComp-BCR /  Β-Carotene Β-Carotene#30: Chemical | ChemComp-SQD / #31: Chemical | ChemComp-LMG / #33: Chemical |  Bicarbonate Bicarbonate#34: Chemical |  Plastoquinone Plastoquinone#35: Chemical | ChemComp-LHG /  Phosphatidylglycerol Phosphatidylglycerol#36: Chemical |  Heme B Heme B#37: Chemical | ChemComp-CHL /  Chlorophyll b Chlorophyll b#38: Chemical | ChemComp-LUT / (  Lutein Lutein#39: Chemical | ChemComp-XAT / (  Violaxanthin Violaxanthin#40: Chemical | ChemComp-NEX / ( |
|---|
 Movie
Movie Controller
Controller








 PDBj
PDBj
























