[English] 日本語
 Yorodumi
Yorodumi- PDB-3f3r: Crystal structure of yeast Thioredoxin1-glutathione mixed disulfi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3f3r | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of yeast Thioredoxin1-glutathione mixed disulfide complex | ||||||
 Components Components | Thioredoxin-1 | ||||||
 Keywords Keywords |  ELECTRON TRANSPORT / ELECTRON TRANSPORT /  thioredoxin / thioredoxin /  glutathione / Deoxyribonucleotide synthesis / glutathione / Deoxyribonucleotide synthesis /  Golgi apparatus / Golgi apparatus /  Membrane / Membrane /  Nucleus / Nucleus /  Protein transport / Redox-active center / Protein transport / Redox-active center /  Phosphoprotein / Transport Phosphoprotein / Transport | ||||||
| Function / homology |  Function and homology information Function and homology informationmembrane fusion priming complex / protein deglutathionylation / vacuole inheritance / vacuole fusion, non-autophagic / disulfide oxidoreductase activity / fungal-type vacuole / retrograde vesicle-mediated transport, Golgi to endoplasmic reticulum / deoxyribonucleotide biosynthetic process /  protein-disulfide reductase activity / endoplasmic reticulum to Golgi vesicle-mediated transport ...membrane fusion priming complex / protein deglutathionylation / vacuole inheritance / vacuole fusion, non-autophagic / disulfide oxidoreductase activity / fungal-type vacuole / retrograde vesicle-mediated transport, Golgi to endoplasmic reticulum / deoxyribonucleotide biosynthetic process / protein-disulfide reductase activity / endoplasmic reticulum to Golgi vesicle-mediated transport ...membrane fusion priming complex / protein deglutathionylation / vacuole inheritance / vacuole fusion, non-autophagic / disulfide oxidoreductase activity / fungal-type vacuole / retrograde vesicle-mediated transport, Golgi to endoplasmic reticulum / deoxyribonucleotide biosynthetic process /  protein-disulfide reductase activity / endoplasmic reticulum to Golgi vesicle-mediated transport / cell redox homeostasis / protein-disulfide reductase activity / endoplasmic reticulum to Golgi vesicle-mediated transport / cell redox homeostasis /  mitochondrial intermembrane space / mitochondrial intermembrane space /  protein transport / protein transport /  Golgi membrane / Golgi membrane /  mitochondrion / mitochondrion /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Zhang, Y.R. / Bao, R. / Zhou, C.Z. / Chen, Y.X. | ||||||
 Citation Citation |  Journal: Biochim.Biophys.Acta / Year: 2009 Journal: Biochim.Biophys.Acta / Year: 2009Title: Structural and kinetic analysis of Saccharomyces cerevisiae thioredoxin Trx1: implications for the catalytic mechanism of GSSG reduced by the thioredoxin system Authors: Bao, R. / Zhang, Y.R. / Lou, X. / Zhou, C.Z. / Chen, Y.X. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3f3r.cif.gz 3f3r.cif.gz | 55.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3f3r.ent.gz pdb3f3r.ent.gz | 38.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3f3r.json.gz 3f3r.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f3/3f3r https://data.pdbj.org/pub/pdb/validation_reports/f3/3f3r ftp://data.pdbj.org/pub/pdb/validation_reports/f3/3f3r ftp://data.pdbj.org/pub/pdb/validation_reports/f3/3f3r | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3f3qC  2fa4S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||
| 2 | 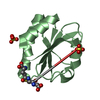
| ||||||||||||||||||
| 3 |
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Ens-ID: 1 / Beg auth comp-ID: MET / Beg label comp-ID: MET / End auth comp-ID: ALA / End label comp-ID: ALA / Refine code: 4 / Auth seq-ID: 1 - 103 / Label seq-ID: 7 - 109
|
- Components
Components
| #1: Protein |  / Thioredoxin I / TR-I / Thioredoxin-2 / Thioredoxin I / TR-I / Thioredoxin-2Mass: 12057.748 Da / Num. of mol.: 2 / Mutation: C33S Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast)Gene: TRX1 / Plasmid: p28 / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: P22217 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: P22217#2: Chemical |  Glutathione Glutathione#3: Chemical |  Sulfate Sulfate#4: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.21 Å3/Da / Density % sol: 44.42 % |
|---|---|
Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 4.4 Details: 2.2M Ammonium Sulfate, 0.1M Sodium Acetate, pH 4.4, VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU MICROMAX-007 / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU MICROMAX-007 / Wavelength: 1.5418 Å |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Jun 3, 2008 / Details: mirrors |
| Radiation | Monochromator: GRAPHITE / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→33.31 Å / Num. all: 17174 / Num. obs: 17174 / % possible obs: 92.73 % / Observed criterion σ(F): 1 / Observed criterion σ(I): 1 / Redundancy: 3.9 % / Biso Wilson estimate: 13.7 Å2 / Rmerge(I) obs: 0.033 / Net I/σ(I): 31.9 |
| Reflection shell | Resolution: 1.8→1.9 Å / Redundancy: 3.8 % / Rmerge(I) obs: 0.057 / Mean I/σ(I) obs: 22.5 / Num. unique all: 2416 / % possible all: 89.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2FA4 Resolution: 1.8→30 Å / Cor.coef. Fo:Fc: 0.939 / Cor.coef. Fo:Fc free: 0.915 / SU B: 4.762 / SU ML: 0.079 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.165 / ESU R Free: 0.144 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 4.19 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→30 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS | Dom-ID: 1 / Auth asym-ID: A / Ens-ID: 1 / Number: 778 / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.8→1.847 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller











 PDBj
PDBj








