Entry Database : PDB / ID : 3edzTitle Crystal structure of catalytic domain of TACE with hydroxamate inhibitor ADAM 17 Keywords / / / / / / / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 1.9 Å Authors Mazzola, R.D. / Zhu, Z. / Sinning, L. / McKittrick, B. / Lavey, B. / Spitler, J. / Kozlowski, J. / Neng-Yang, S. / Zhou, G. / Guo, Z. ...Mazzola, R.D. / Zhu, Z. / Sinning, L. / McKittrick, B. / Lavey, B. / Spitler, J. / Kozlowski, J. / Neng-Yang, S. / Zhou, G. / Guo, Z. / Orth, P. / Madison, V. / Sun, J. / Lundell, D. / Niu, X. Journal : Bioorg.Med.Chem.Lett. / Year : 2008Title : Discovery of novel hydroxamates as highly potent tumor necrosis factor-alpha converting enzyme inhibitors. Part II: optimization of the S3' pocket.Authors : Mazzola, R.D. / Zhu, Z. / Sinning, L. / McKittrick, B. / Lavey, B. / Spitler, J. / Kozlowski, J. / Neng-Yang, S. / Zhou, G. / Guo, Z. / Orth, P. / Madison, V. / Sun, J. / Lundell, D. / Niu, X. History Deposition Sep 3, 2008 Deposition site / Processing site Revision 1.0 Sep 23, 2008 Provider / Type Revision 1.1 Jul 13, 2011 Group / Version format complianceRevision 1.2 Feb 27, 2013 Group Revision 1.3 Oct 20, 2021 Group / Derived calculationsCategory database_2 / pdbx_struct_conn_angle ... database_2 / pdbx_struct_conn_angle / struct_conn / struct_ref_seq_dif / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components
 Keywords
Keywords HYDROLASE / ZN-ENDOPEPTIDASE / Cleavage on pair of basic residues /
HYDROLASE / ZN-ENDOPEPTIDASE / Cleavage on pair of basic residues /  Glycoprotein /
Glycoprotein /  Membrane / Metal-binding /
Membrane / Metal-binding /  Metalloprotease /
Metalloprotease /  Notch signaling pathway /
Notch signaling pathway /  Phosphoprotein /
Phosphoprotein /  Protease / SH3-binding /
Protease / SH3-binding /  Transmembrane /
Transmembrane /  Zymogen
Zymogen Function and homology information
Function and homology information ADAM 17 endopeptidase / regulation of mast cell apoptotic process / metalloendopeptidase activity involved in amyloid precursor protein catabolic process / cellular response to high density lipoprotein particle stimulus / positive regulation of epidermal growth factor-activated receptor activity / Constitutive Signaling by NOTCH1 t(7;9)(NOTCH1:M1580_K2555) Translocation Mutant / Notch receptor processing /
ADAM 17 endopeptidase / regulation of mast cell apoptotic process / metalloendopeptidase activity involved in amyloid precursor protein catabolic process / cellular response to high density lipoprotein particle stimulus / positive regulation of epidermal growth factor-activated receptor activity / Constitutive Signaling by NOTCH1 t(7;9)(NOTCH1:M1580_K2555) Translocation Mutant / Notch receptor processing /  interleukin-6 receptor binding /
interleukin-6 receptor binding /  tumor necrosis factor binding / positive regulation of T cell chemotaxis ...
tumor necrosis factor binding / positive regulation of T cell chemotaxis ... ADAM 17 endopeptidase / regulation of mast cell apoptotic process / metalloendopeptidase activity involved in amyloid precursor protein catabolic process / cellular response to high density lipoprotein particle stimulus / positive regulation of epidermal growth factor-activated receptor activity / Constitutive Signaling by NOTCH1 t(7;9)(NOTCH1:M1580_K2555) Translocation Mutant / Notch receptor processing /
ADAM 17 endopeptidase / regulation of mast cell apoptotic process / metalloendopeptidase activity involved in amyloid precursor protein catabolic process / cellular response to high density lipoprotein particle stimulus / positive regulation of epidermal growth factor-activated receptor activity / Constitutive Signaling by NOTCH1 t(7;9)(NOTCH1:M1580_K2555) Translocation Mutant / Notch receptor processing /  interleukin-6 receptor binding /
interleukin-6 receptor binding /  tumor necrosis factor binding / positive regulation of T cell chemotaxis / TNF signaling / germinal center formation / Regulated proteolysis of p75NTR / Release of Hh-Np from the secreting cell / commissural neuron axon guidance / positive regulation of tumor necrosis factor-mediated signaling pathway / neutrophil mediated immunity /
tumor necrosis factor binding / positive regulation of T cell chemotaxis / TNF signaling / germinal center formation / Regulated proteolysis of p75NTR / Release of Hh-Np from the secreting cell / commissural neuron axon guidance / positive regulation of tumor necrosis factor-mediated signaling pathway / neutrophil mediated immunity /  wound healing, spreading of epidermal cells / Notch binding / negative regulation of cold-induced thermogenesis / positive regulation of leukocyte chemotaxis / CD163 mediating an anti-inflammatory response / positive regulation of vascular endothelial cell proliferation / positive regulation of epidermal growth factor receptor signaling pathway / cell adhesion mediated by integrin / Signaling by EGFR / amyloid precursor protein catabolic process /
wound healing, spreading of epidermal cells / Notch binding / negative regulation of cold-induced thermogenesis / positive regulation of leukocyte chemotaxis / CD163 mediating an anti-inflammatory response / positive regulation of vascular endothelial cell proliferation / positive regulation of epidermal growth factor receptor signaling pathway / cell adhesion mediated by integrin / Signaling by EGFR / amyloid precursor protein catabolic process /  membrane protein ectodomain proteolysis / Collagen degradation / positive regulation of cyclin-dependent protein serine/threonine kinase activity / positive regulation of G1/S transition of mitotic cell cycle / positive regulation of blood vessel endothelial cell migration / Growth hormone receptor signaling / Nuclear signaling by ERBB4 / positive regulation of chemokine production /
membrane protein ectodomain proteolysis / Collagen degradation / positive regulation of cyclin-dependent protein serine/threonine kinase activity / positive regulation of G1/S transition of mitotic cell cycle / positive regulation of blood vessel endothelial cell migration / Growth hormone receptor signaling / Nuclear signaling by ERBB4 / positive regulation of chemokine production /  Notch signaling pathway / spleen development / Constitutive Signaling by NOTCH1 HD Domain Mutants / Activated NOTCH1 Transmits Signal to the Nucleus / B cell differentiation /
Notch signaling pathway / spleen development / Constitutive Signaling by NOTCH1 HD Domain Mutants / Activated NOTCH1 Transmits Signal to the Nucleus / B cell differentiation /  PDZ domain binding /
PDZ domain binding /  cell motility / negative regulation of transforming growth factor beta receptor signaling pathway / protein processing /
cell motility / negative regulation of transforming growth factor beta receptor signaling pathway / protein processing /  metalloendopeptidase activity / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants /
metalloendopeptidase activity / Constitutive Signaling by NOTCH1 PEST Domain Mutants / Constitutive Signaling by NOTCH1 HD+PEST Domain Mutants /  SH3 domain binding /
SH3 domain binding /  metallopeptidase activity /
metallopeptidase activity /  actin cytoskeleton /
actin cytoskeleton /  integrin binding / T cell differentiation in thymus /
integrin binding / T cell differentiation in thymus /  peptidase activity / positive regulation of cell growth /
peptidase activity / positive regulation of cell growth /  endopeptidase activity / response to lipopolysaccharide / response to hypoxia /
endopeptidase activity / response to lipopolysaccharide / response to hypoxia /  cell adhesion / positive regulation of cell migration / defense response to Gram-positive bacterium / response to xenobiotic stimulus / apical plasma membrane /
cell adhesion / positive regulation of cell migration / defense response to Gram-positive bacterium / response to xenobiotic stimulus / apical plasma membrane /  membrane raft /
membrane raft /  endoplasmic reticulum lumen /
endoplasmic reticulum lumen /  Golgi membrane / positive regulation of cell population proliferation /
Golgi membrane / positive regulation of cell population proliferation /  cell surface /
cell surface /  proteolysis /
proteolysis /  membrane /
membrane /  metal ion binding /
metal ion binding /  plasma membrane /
plasma membrane /  cytosol /
cytosol /  cytoplasm
cytoplasm
 Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON / PDB entry 1bkc / Resolution: 1.9 Å
SYNCHROTRON / PDB entry 1bkc / Resolution: 1.9 Å  Authors
Authors Citation
Citation Journal: Bioorg.Med.Chem.Lett. / Year: 2008
Journal: Bioorg.Med.Chem.Lett. / Year: 2008 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 3edz.cif.gz
3edz.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb3edz.ent.gz
pdb3edz.ent.gz PDB format
PDB format 3edz.json.gz
3edz.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ed/3edz
https://data.pdbj.org/pub/pdb/validation_reports/ed/3edz ftp://data.pdbj.org/pub/pdb/validation_reports/ed/3edz
ftp://data.pdbj.org/pub/pdb/validation_reports/ed/3edz Links
Links Assembly
Assembly


 Components
Components / A disintegrin and metalloproteinase domain 17 / TNF-alpha-converting enzyme / TNF-alpha convertase ...A disintegrin and metalloproteinase domain 17 / TNF-alpha-converting enzyme / TNF-alpha convertase / Snake venom-like protease
/ A disintegrin and metalloproteinase domain 17 / TNF-alpha-converting enzyme / TNF-alpha convertase ...A disintegrin and metalloproteinase domain 17 / TNF-alpha-converting enzyme / TNF-alpha convertase / Snake venom-like protease
 Homo sapiens (human) / Gene: ADAM17, CSVP, TACE / Production host:
Homo sapiens (human) / Gene: ADAM17, CSVP, TACE / Production host: 
 Trichoplusia ni (cabbage looper) / References: UniProt: P78536,
Trichoplusia ni (cabbage looper) / References: UniProt: P78536,  ADAM 17 endopeptidase
ADAM 17 endopeptidase
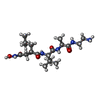







 Citric acid
Citric acid Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 17-ID / Wavelength: 1 Å
/ Beamline: 17-ID / Wavelength: 1 Å : 1 Å / Relative weight: 1
: 1 Å / Relative weight: 1  Processing
Processing : PDB entry 1bkc / Resolution: 1.9→28.83 Å / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
: PDB entry 1bkc / Resolution: 1.9→28.83 Å / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber Movie
Movie Controller
Controller














 PDBj
PDBj














