[English] 日本語
 Yorodumi
Yorodumi- PDB-2zis: Crystal Structure of rat protein farnesyltransferase complexed wi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2zis | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of rat protein farnesyltransferase complexed with a bezoruran inhibitor and FPP | ||||||
 Components Components | (Protein farnesyltransferase subunit ... Farnesyltransferase) x 2 Farnesyltransferase) x 2 | ||||||
 Keywords Keywords |  TRANSFERASE / TRANSFERASE /  Prenyltransferase / Metal-binding / Prenyltransferase / Metal-binding /  Phosphoprotein Phosphoprotein | ||||||
| Function / homology |  Function and homology information Function and homology informationApoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex /  protein geranylgeranyltransferase type I / protein farnesylation / positive regulation of tubulin deacetylation / protein geranylgeranyltransferase type I / protein farnesylation / positive regulation of tubulin deacetylation /  protein farnesyltransferase ...Apoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex / protein farnesyltransferase ...Apoptotic cleavage of cellular proteins / Inactivation, recovery and regulation of the phototransduction cascade / RAS processing / protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase activity / CAAX-protein geranylgeranyltransferase complex /  protein geranylgeranyltransferase type I / protein farnesylation / positive regulation of tubulin deacetylation / protein geranylgeranyltransferase type I / protein farnesylation / positive regulation of tubulin deacetylation /  protein farnesyltransferase / protein farnesyltransferase /  protein farnesyltransferase activity / protein farnesyltransferase activity /  protein farnesyltransferase complex / regulation of fibroblast proliferation / protein farnesyltransferase complex / regulation of fibroblast proliferation /  Rab geranylgeranyltransferase activity / protein geranylgeranylation / positive regulation of skeletal muscle acetylcholine-gated channel clustering / Rab geranylgeranyltransferase activity / protein geranylgeranylation / positive regulation of skeletal muscle acetylcholine-gated channel clustering /  farnesyltranstransferase activity / negative regulation of nitric-oxide synthase biosynthetic process / microtubule associated complex / farnesyltranstransferase activity / negative regulation of nitric-oxide synthase biosynthetic process / microtubule associated complex /  enzyme-linked receptor protein signaling pathway / positive regulation of nitric-oxide synthase biosynthetic process / positive regulation of Rac protein signal transduction / alpha-tubulin binding / response to inorganic substance / positive regulation of cell cycle / response to cytokine / enzyme-linked receptor protein signaling pathway / positive regulation of nitric-oxide synthase biosynthetic process / positive regulation of Rac protein signal transduction / alpha-tubulin binding / response to inorganic substance / positive regulation of cell cycle / response to cytokine /  wound healing / lipid metabolic process / response to organic cyclic compound / wound healing / lipid metabolic process / response to organic cyclic compound /  receptor tyrosine kinase binding / positive regulation of fibroblast proliferation / fibroblast proliferation / receptor tyrosine kinase binding / positive regulation of fibroblast proliferation / fibroblast proliferation /  microtubule binding / cell population proliferation / molecular adaptor activity / negative regulation of cell population proliferation / positive regulation of cell population proliferation / negative regulation of apoptotic process / zinc ion binding / microtubule binding / cell population proliferation / molecular adaptor activity / negative regulation of cell population proliferation / positive regulation of cell population proliferation / negative regulation of apoptotic process / zinc ion binding /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  molecular replacement / Resolution: 2.6 Å molecular replacement / Resolution: 2.6 Å | ||||||
 Authors Authors | Fukami, T.A. / Sogabe, S. / Nagata, Y. / Kondoh, O. / Ishii, N. | ||||||
 Citation Citation |  Journal: Bioorg.Med.Chem.Lett. / Year: 2009 Journal: Bioorg.Med.Chem.Lett. / Year: 2009Title: Synthesis and structure-activity relationships of novel benzofuran farnesyltransferase inhibitors Authors: Asoh, K. / Kohchi, M. / Hyoudoh, I. / Ohtsuka, T. / Masubuchi, M. / Kawasaki, K. / Ebiike, H. / Shiratori, Y. / Fukami, T.A. / Kondoh, O. / Tsukaguchi, T. / Ishii, N. / Aoki, Y. / Shimma, N. / Sakaitani, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2zis.cif.gz 2zis.cif.gz | 173.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2zis.ent.gz pdb2zis.ent.gz | 132.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2zis.json.gz 2zis.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zi/2zis https://data.pdbj.org/pub/pdb/validation_reports/zi/2zis ftp://data.pdbj.org/pub/pdb/validation_reports/zi/2zis ftp://data.pdbj.org/pub/pdb/validation_reports/zi/2zis | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2zirC  1fppS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein farnesyltransferase subunit ... , 2 types, 2 molecules AB
| #1: Protein |  Farnesyltransferase / CAAX farnesyltransferase subunit alpha / Ras proteins prenyltransferase alpha / FTase-alpha / Type ...CAAX farnesyltransferase subunit alpha / Ras proteins prenyltransferase alpha / FTase-alpha / Type I protein geranyl-geranyltransferase subunit alpha / GGTase-I-alpha Farnesyltransferase / CAAX farnesyltransferase subunit alpha / Ras proteins prenyltransferase alpha / FTase-alpha / Type ...CAAX farnesyltransferase subunit alpha / Ras proteins prenyltransferase alpha / FTase-alpha / Type I protein geranyl-geranyltransferase subunit alpha / GGTase-I-alphaMass: 44086.090 Da / Num. of mol.: 1 / Mutation: I156T Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Fnta / Plasmid: pET14b / Production host: Rattus norvegicus (Norway rat) / Gene: Fnta / Plasmid: pET14b / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3)CP / References: UniProt: Q04631, Escherichia coli (E. coli) / Strain (production host): BL21(DE3)CP / References: UniProt: Q04631,  protein farnesyltransferase protein farnesyltransferase |
|---|---|
| #2: Protein |  Farnesyltransferase / CAAX farnesyltransferase subunit beta / RAS proteins prenyltransferase beta / FTase-beta Farnesyltransferase / CAAX farnesyltransferase subunit beta / RAS proteins prenyltransferase beta / FTase-betaMass: 49004.562 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Fntb / Plasmid: pET14b / Production host: Rattus norvegicus (Norway rat) / Gene: Fntb / Plasmid: pET14b / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3)CP / References: UniProt: Q02293, Escherichia coli (E. coli) / Strain (production host): BL21(DE3)CP / References: UniProt: Q02293,  protein farnesyltransferase protein farnesyltransferase |
-Non-polymers , 6 types, 411 molecules 

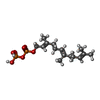








| #3: Chemical |  Acetic acid Acetic acid#4: Chemical | ChemComp-ZN / | #5: Chemical | ChemComp-FPP / |  Farnesyl pyrophosphate Farnesyl pyrophosphate#6: Chemical | ChemComp-NH8 / | #7: Chemical |  Glycerol Glycerol#8: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.2 Å3/Da / Density % sol: 60.8 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 4.5 Details: 20mg/ml protein, 0.5mM FPP, 1mM inhibitor, 10% PEG6000, 0.2M Mg acetate, 0.1M Na acetate buffer, pH4.5, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Photon Factory Photon Factory  / Beamline: BL-6B / Wavelength: 1 Å / Beamline: BL-6B / Wavelength: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: WEISSENBERG / Detector: DIFFRACTOMETER / Date: Jun 5, 2001 / Details: mirrors | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Si(111) double-crystal / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.6→100 Å / Num. obs: 35232 / % possible obs: 97.6 % / Rmerge(I) obs: 0.069 / Χ2: 1.048 / Net I/σ(I): 16.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
-Phasing
Phasing | Method:  molecular replacement molecular replacement | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MR | Rfactor: 0.316 / Cor.coef. Fo:Fc: 0.769
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  molecular replacement molecular replacementStarting model: PDB ENTRY 1FPP Resolution: 2.6→42.96 Å / FOM work R set: 0.855 / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 37.178 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.6→42.96 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 35
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj








