[English] 日本語
 Yorodumi
Yorodumi- PDB-1uhm: Solution structure of the globular domain of linker histone homol... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1uhm | ||||||
|---|---|---|---|---|---|---|---|
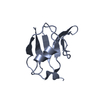
| Title | Solution structure of the globular domain of linker histone homolog Hho1p from S. cerevisiae | ||||||
 Components Components | Histone H1 | ||||||
 Keywords Keywords |  STRUCTURAL PROTEIN / winged helix-turn-helix / linker histone / STRUCTURAL PROTEIN / winged helix-turn-helix / linker histone /  S. cerevisiae / RIKEN Structural Genomics/Proteomics Initiative / RSGI / S. cerevisiae / RIKEN Structural Genomics/Proteomics Initiative / RSGI /  Structural Genomics Structural Genomics | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of DNA recombination /  supercoiled DNA binding / chromosome condensation / supercoiled DNA binding / chromosome condensation /  phosphate ion binding / nucleosomal DNA binding / phosphate ion binding / nucleosomal DNA binding /  protein-DNA complex / chromatin DNA binding / structural constituent of chromatin / protein-DNA complex / chromatin DNA binding / structural constituent of chromatin /  nucleosome / nucleosome /  nucleosome assembly ...negative regulation of DNA recombination / nucleosome assembly ...negative regulation of DNA recombination /  supercoiled DNA binding / chromosome condensation / supercoiled DNA binding / chromosome condensation /  phosphate ion binding / nucleosomal DNA binding / phosphate ion binding / nucleosomal DNA binding /  protein-DNA complex / chromatin DNA binding / structural constituent of chromatin / protein-DNA complex / chromatin DNA binding / structural constituent of chromatin /  nucleosome / nucleosome /  nucleosome assembly / nucleosome assembly /  double-stranded DNA binding / double-stranded DNA binding /  nucleic acid binding / molecular adaptor activity / nucleic acid binding / molecular adaptor activity /  chromatin / regulation of DNA-templated transcription / chromatin / regulation of DNA-templated transcription /  DNA binding / DNA binding /  nucleus nucleusSimilarity search - Function | ||||||
| Biological species |   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast) | ||||||
| Method |  SOLUTION NMR / distance geometry simulated annealing, SOLUTION NMR / distance geometry simulated annealing,  molecular dynamics molecular dynamics | ||||||
 Authors Authors | Ono, K. / Kusano, O. / Shimotakahara, S. / Shimizu, M. / Yamazaki, T. / Shindo, H. / RIKEN Structural Genomics/Proteomics Initiative (RSGI) | ||||||
 Citation Citation |  Journal: Nucleic Acids Res. / Year: 2003 Journal: Nucleic Acids Res. / Year: 2003Title: The linker histone homolog Hho1p from Saccharomyces cerevisiae represents a winged helix-turn-helix fold as determined by NMR spectroscopy. Authors: Ono, K. / Kusano, O. / Shimotakahara, S. / Shimizu, M. / Yamazaki, T. / Shindo, H. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1uhm.cif.gz 1uhm.cif.gz | 475.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1uhm.ent.gz pdb1uhm.ent.gz | 396.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1uhm.json.gz 1uhm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uh/1uhm https://data.pdbj.org/pub/pdb/validation_reports/uh/1uhm ftp://data.pdbj.org/pub/pdb/validation_reports/uh/1uhm ftp://data.pdbj.org/pub/pdb/validation_reports/uh/1uhm | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein |  / Histone Hho1p / Histone Hho1pMass: 8516.781 Da / Num. of mol.: 1 / Fragment: globular domain Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Saccharomyces cerevisiae (brewer's yeast) Saccharomyces cerevisiae (brewer's yeast)Gene: HHO1 / Plasmid: pET21b / Species (production host): Escherichia coli / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: P53551 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: P53551 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR |
|---|
- Sample preparation
Sample preparation
| Details | Contents: 1mM HD1 U-15N,13C; 10mM phosphate buffer / Solvent system: 90% H2O/10% D2O |
|---|---|
| Sample conditions | pH: 6.2 / Pressure: ambient / Temperature: 298 K |
Crystal grow | *PLUS Method: other / Details: NMR |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| NMR spectrometer | Type: Bruker DMX / Manufacturer: Bruker / Model : DMX / Field strength: 750 MHz : DMX / Field strength: 750 MHz |
- Processing
Processing
| NMR software | Name:  X-PLOR / Version: 3.1 / Developer: Brunger, A.T. / Classification: refinement X-PLOR / Version: 3.1 / Developer: Brunger, A.T. / Classification: refinement |
|---|---|
| Refinement | Method: distance geometry simulated annealing,  molecular dynamics molecular dynamicsSoftware ordinal: 1 |
| NMR ensemble | Conformer selection criteria: structures with acceptable covalent geometry, structures with favorable non-bond energy, structures with the least restraint violations, structures with the lowest energy Conformers calculated total number: 100 / Conformers submitted total number: 20 |
 Movie
Movie Controller
Controller








 PDBj
PDBj