+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1q5f | ||||||
|---|---|---|---|---|---|---|---|
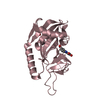
| Title | NMR Structure of Type IVb pilin (PilS) from Salmonella typhi | ||||||
 Components Components | PilS | ||||||
 Keywords Keywords |  CELL ADHESION / TYPE IVb PILIN / ALPHA-BETA ROLL / CELL ADHESION / TYPE IVb PILIN / ALPHA-BETA ROLL /  MONOMER MONOMER | ||||||
| Function / homology | Type 4 secretion system, PilS, N-terminal / PilS N terminal / TcpA-like pilin / TcpA-like pilin / membrane => GO:0016020 / 2-Layer Sandwich / Alpha Beta /  PilS PilS Function and homology information Function and homology information | ||||||
| Biological species |   Salmonella typhi (bacteria) Salmonella typhi (bacteria) | ||||||
| Method |  SOLUTION NMR / distance geometry, SOLUTION NMR / distance geometry,  simulated annealing simulated annealing | ||||||
 Authors Authors | Xu, X.F. / Tan, Y.W. / Hackett, J. / Zhang, M. / Mok, Y.K. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2004 Journal: J.Biol.Chem. / Year: 2004Title: NMR Structure of a Type IVb Pilin from Salmonella typhi and Its Assembly into Pilus Authors: Xu, X.F. / Tan, Y.W. / Lam, L. / Hackett, J. / Zhang, M. / Mok, Y.K. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1q5f.cif.gz 1q5f.cif.gz | 399.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1q5f.ent.gz pdb1q5f.ent.gz | 343.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1q5f.json.gz 1q5f.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/q5/1q5f https://data.pdbj.org/pub/pdb/validation_reports/q5/1q5f ftp://data.pdbj.org/pub/pdb/validation_reports/q5/1q5f ftp://data.pdbj.org/pub/pdb/validation_reports/q5/1q5f | HTTPS FTP |
|---|
-Related structure data
| Similar structure data | |
|---|---|
| Other databases |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||
| NMR ensembles |
|
- Components
Components
| #1: Protein |  / Type IVb pilin / Type IVb pilinMass: 15842.432 Da / Num. of mol.: 1 / Fragment: RESIDUES 26-181 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Salmonella typhi (bacteria) / Gene: PilS / Plasmid: pET-H / Species (production host): Escherichia coli / Production host: Salmonella typhi (bacteria) / Gene: PilS / Plasmid: pET-H / Species (production host): Escherichia coli / Production host:   Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: Q9ZIU9 Escherichia coli BL21(DE3) (bacteria) / Strain (production host): BL21(DE3) / References: UniProt: Q9ZIU9 |
|---|
-Experimental details
-Experiment
| Experiment | Method:  SOLUTION NMR SOLUTION NMR | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NMR experiment |
| ||||||||||||||||||||
| NMR details | Text: The structures are based on a total of 2315 restraints, 2033 are NOE-derived distance constraints, 206 dihedral angle restraints, 76 distance restraints from hydrogen bonds. |
- Sample preparation
Sample preparation
| Details |
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample conditions | Ionic strength: 0.55 / pH: 6.0 / Pressure: ambient / Temperature: 303 K |
-NMR measurement
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radiation wavelength | Relative weight: 1 | |||||||||||||||
| NMR spectrometer |
|
- Processing
Processing
| NMR software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method: distance geometry,  simulated annealing / Software ordinal: 1 simulated annealing / Software ordinal: 1 | ||||||||||||||||||||||||||||
| NMR representative | Selection criteria: lowest energy | ||||||||||||||||||||||||||||
| NMR ensemble | Conformer selection criteria: structures with the lowest energy Conformers calculated total number: 100 / Conformers submitted total number: 10 |
 Movie
Movie Controller
Controller












 PDBj
PDBj