+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1ijv | ||||||
|---|---|---|---|---|---|---|---|
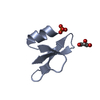
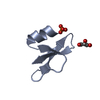
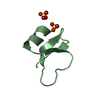
| Title | HUMAN BETA-DEFENSIN-1 | ||||||
 Components Components | Beta-defensin 1 Beta defensin Beta defensin | ||||||
 Keywords Keywords |  DEFENSIN / DEFENSIN /  HUMAN BETA-DEFENSIN-1 / HUMAN BETA-DEFENSIN-1 /  BETA-DEFENSIN BETA-DEFENSIN | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of flagellated sperm motility involved in capacitation /  microvesicle / CCR6 chemokine receptor binding / microvesicle / CCR6 chemokine receptor binding /  Beta defensins / Beta defensins /  Defensins / cAMP-mediated signaling / : / sperm midpiece / Defensins / cAMP-mediated signaling / : / sperm midpiece /  innate immune response in mucosa / response to bacterium ...positive regulation of flagellated sperm motility involved in capacitation / innate immune response in mucosa / response to bacterium ...positive regulation of flagellated sperm motility involved in capacitation /  microvesicle / CCR6 chemokine receptor binding / microvesicle / CCR6 chemokine receptor binding /  Beta defensins / Beta defensins /  Defensins / cAMP-mediated signaling / : / sperm midpiece / Defensins / cAMP-mediated signaling / : / sperm midpiece /  innate immune response in mucosa / response to bacterium / Golgi lumen / innate immune response in mucosa / response to bacterium / Golgi lumen /  chemotaxis / antimicrobial humoral immune response mediated by antimicrobial peptide / antibacterial humoral response / defense response to Gram-negative bacterium / defense response to Gram-positive bacterium / defense response to bacterium / chemotaxis / antimicrobial humoral immune response mediated by antimicrobial peptide / antibacterial humoral response / defense response to Gram-negative bacterium / defense response to Gram-positive bacterium / defense response to bacterium /  immune response / G protein-coupled receptor signaling pathway / immune response / G protein-coupled receptor signaling pathway /  innate immune response / innate immune response /  extracellular space / extracellular exosome / extracellular region / extracellular space / extracellular exosome / extracellular region /  membrane / identical protein binding membrane / identical protein bindingSimilarity search - Function | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  AB INITIO / Resolution: 1.2 Å AB INITIO / Resolution: 1.2 Å | ||||||
 Authors Authors | Hoover, D.M. / Lubkowski, J. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2001 Journal: J.Biol.Chem. / Year: 2001Title: The structure of human beta-defensin-1: new insights into structural properties of beta-defensins. Authors: Hoover, D.M. / Chertov, O. / Lubkowski, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1ijv.cif.gz 1ijv.cif.gz | 47.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1ijv.ent.gz pdb1ijv.ent.gz | 37.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1ijv.json.gz 1ijv.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ij/1ijv https://data.pdbj.org/pub/pdb/validation_reports/ij/1ijv ftp://data.pdbj.org/pub/pdb/validation_reports/ij/1ijv ftp://data.pdbj.org/pub/pdb/validation_reports/ij/1ijv | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein/peptide |  Beta defensin / HBD-1 Beta defensin / HBD-1Mass: 3940.598 Da / Num. of mol.: 2 / Source method: obtained synthetically Details: THIS PEPTIDE WAS CHEMICALLY SYNTHESIZED. THE SEQUENCE OF THIS PEPTIDE OCCURS NATURALLY IN HUMANS (HOMO SAPIENS). References: UniProt: P60022 #2: Chemical |  Sulfate Sulfate#3: Chemical | ChemComp-BR /  Bromide Bromide#4: Chemical | ChemComp-K / | #5: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.75 Å3/Da / Density % sol: 29.8 % / Description: ANOMALOUS DATA USED FOR REFINEMENT |
|---|---|
Crystal grow | Temperature: 285 K / Method: vapor diffusion, hanging drop / pH: 4.6 Details: PEG 4000, ammonium sulfate, sodium acetate, potassium bromide, glycerol, pH 4.6, VAPOR DIFFUSION, HANGING DROP, temperature 285K |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X9B / Wavelength: 0.92021 / Wavelength: 0.92 Å / Beamline: X9B / Wavelength: 0.92021 / Wavelength: 0.92 Å | |||||||||
| Detector | Type: ADSC / Detector: CCD / Date: Mar 4, 2001 / Details: MIRRORS | |||||||||
| Radiation | Monochromator: SI CRYSTAL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||
| Radiation wavelength |
| |||||||||
| Reflection | Resolution: 1.2→25 Å / Num. all: 87673 / Num. obs: 40500 / % possible obs: 96.4 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 2.2 % / Biso Wilson estimate: 10 Å2 / Rmerge(I) obs: 0.033 / Net I/σ(I): 23.5 | |||||||||
| Reflection shell | Resolution: 1.2→1.24 Å / Redundancy: 2.1 % / Rmerge(I) obs: 0.227 / Mean I/σ(I) obs: 3 / % possible all: 86.4 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  AB INITIO / Resolution: 1.2→20 Å / Num. parameters: 7167 / Num. restraintsaints: 9055 / Cross valid method: FREE R / σ(F): 0 / σ(I): 0 / Stereochemistry target values: ENGH AND HUBER AB INITIO / Resolution: 1.2→20 Å / Num. parameters: 7167 / Num. restraintsaints: 9055 / Cross valid method: FREE R / σ(F): 0 / σ(I): 0 / Stereochemistry target values: ENGH AND HUBER
| |||||||||||||||||||||||||||||||||
| Refine analyze | Occupancy sum hydrogen: 536 / Occupancy sum non hydrogen: 738 | |||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.2→20 Å
| |||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj










