[English] 日本語
 Yorodumi
Yorodumi- PDB-1e8s: Alu domain of the mammalian SRP (potential Alu retroposition inte... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1e8s | ||||||
|---|---|---|---|---|---|---|---|
| Title | Alu domain of the mammalian SRP (potential Alu retroposition intermediate) | ||||||
 Components Components |
| ||||||
 Keywords Keywords | ALU RIBONUCLEOPROTEIN PARTICLE / ALU RNP ASSEMBLY AND DIMERISATION / TRANSLATIONAL CONTROL / ALU RETROPOSITION | ||||||
| Function / homology |  Function and homology information Function and homology information signal recognition particle receptor complex / endoplasmic reticulum signal peptide binding / signal recognition particle receptor complex / endoplasmic reticulum signal peptide binding /  signal recognition particle, endoplasmic reticulum targeting / signal recognition particle, endoplasmic reticulum targeting /  signal recognition particle binding / negative regulation of translational elongation / cotranslational protein targeting to membrane / protein targeting to ER / 7S RNA binding / SRP-dependent cotranslational protein targeting to membrane / SRP-dependent cotranslational protein targeting to membrane ... signal recognition particle binding / negative regulation of translational elongation / cotranslational protein targeting to membrane / protein targeting to ER / 7S RNA binding / SRP-dependent cotranslational protein targeting to membrane / SRP-dependent cotranslational protein targeting to membrane ... signal recognition particle receptor complex / endoplasmic reticulum signal peptide binding / signal recognition particle receptor complex / endoplasmic reticulum signal peptide binding /  signal recognition particle, endoplasmic reticulum targeting / signal recognition particle, endoplasmic reticulum targeting /  signal recognition particle binding / negative regulation of translational elongation / cotranslational protein targeting to membrane / protein targeting to ER / 7S RNA binding / SRP-dependent cotranslational protein targeting to membrane / SRP-dependent cotranslational protein targeting to membrane / secretory granule lumen / ficolin-1-rich granule lumen / Neutrophil degranulation / signal recognition particle binding / negative regulation of translational elongation / cotranslational protein targeting to membrane / protein targeting to ER / 7S RNA binding / SRP-dependent cotranslational protein targeting to membrane / SRP-dependent cotranslational protein targeting to membrane / secretory granule lumen / ficolin-1-rich granule lumen / Neutrophil degranulation /  RNA binding / extracellular region / RNA binding / extracellular region /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 4 Å MAD / Resolution: 4 Å | ||||||
| Model type details | CA ATOMS ONLY, CHAIN A, B ; P ATOMS ONLY, CHAIN C | ||||||
 Authors Authors | Weichenrieder, O. / Wild, K. / Strub, K. / Cusack, S. | ||||||
 Citation Citation |  Journal: Nature / Year: 2000 Journal: Nature / Year: 2000Title: Structure and Assembly of the Alu Domain of the Mammalian Signal Recognition Particle Authors: Weichenrieder, O. / Wild, K. / Strub, K. / Cusack, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1e8s.cif.gz 1e8s.cif.gz | 22.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1e8s.ent.gz pdb1e8s.ent.gz | 9.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1e8s.json.gz 1e8s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e8/1e8s https://data.pdbj.org/pub/pdb/validation_reports/e8/1e8s ftp://data.pdbj.org/pub/pdb/validation_reports/e8/1e8s ftp://data.pdbj.org/pub/pdb/validation_reports/e8/1e8s | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 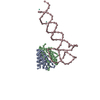
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 9996.567 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   HOMO SAPIENS (human) / Cellular location: CYTOPLASM, NUCLEUS? HOMO SAPIENS (human) / Cellular location: CYTOPLASM, NUCLEUS? / Production host: / Production host:   ESCHERICHIA COLI (E. coli) / References: UniProt: P49458 ESCHERICHIA COLI (E. coli) / References: UniProt: P49458 | ||
|---|---|---|---|
| #2: Protein | Mass: 12114.235 Da / Num. of mol.: 1 / Fragment: TRUNCATED AFTER K107 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   HOMO SAPIENS (human) / Cellular location: CYTOPLASM, NUCLEUS? HOMO SAPIENS (human) / Cellular location: CYTOPLASM, NUCLEUS? / Production host: / Production host:   ESCHERICHIA COLI (E. coli) / References: UniProt: P37108 ESCHERICHIA COLI (E. coli) / References: UniProt: P37108 | ||
| #3: RNA chain | Mass: 28490.900 Da / Num. of mol.: 1 / Fragment: ALU RNA / Mutation: YES / Source method: obtained synthetically Details: THE RNA WAS PRODUCED BY IN VITRO TRANSCRIPTION WITH T7 RNA POLYMERASE USING RIBOZYME TECHNOLOGY. Source: (synth.)   HOMO SAPIENS (human) HOMO SAPIENS (human) | ||
| #4: Chemical |  Europium EuropiumCompound details | SIGNAL-RECOGNITION-PARTICLE ASSEMBLY HAS A CRUCIAL ROLE IN TARGETING SECRETORY PROTEINS TO THE ...SIGNAL-RECOGNITIO | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.06 Å3/Da / Density % sol: 66 % / Description: EUROPIUM L(III) EDGE | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | pH: 7 Details: 50MM HEPES, 10MM MGCL2, 150MM NACL, 0.8 MM EU(NO3)3, 390MM (NH4)2SO4, 23% PEG400, pH 7.00 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal | *PLUS Density % sol: 67 % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 12 ℃ / Method: vapor diffusion, hanging drop / pH: 7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-2 / Wavelength: 1.7757,1.7753,1.033,0.9326 / Beamline: ID14-2 / Wavelength: 1.7757,1.7753,1.033,0.9326 | |||||||||||||||
| Detector | Type: MARRESEARCH / Detector: CCD / Date: Nov 15, 1999 | |||||||||||||||
| Radiation | Protocol: MAD / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||
| Radiation wavelength |
| |||||||||||||||
| Reflection | Resolution: 4→50 Å / Num. obs: 5053 / % possible obs: 90.3 % / Redundancy: 2.9 % / Biso Wilson estimate: 211.4 Å2 / Rsym value: 0.059 / Net I/σ(I): 5.9 | |||||||||||||||
| Reflection shell | Resolution: 4→4.1 Å / Redundancy: 1.9 % / Mean I/σ(I) obs: 1.5 / Rsym value: 0.541 / % possible all: 59.6 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MAD / Resolution: 4→37.46 Å / Data cutoff high absF: 3024867.55 / Isotropic thermal model: RESTRAINED / σ(F): 0 MAD / Resolution: 4→37.46 Å / Data cutoff high absF: 3024867.55 / Isotropic thermal model: RESTRAINED / σ(F): 0
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 15 Å2 / ksol: 0.25 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 50 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 4→37.46 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 1 / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller











 PDBj
PDBj

































