+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1bir | ||||||
|---|---|---|---|---|---|---|---|
| Title | RIBONUCLEASE T1, PHE 100 TO ALA MUTANT COMPLEXED WITH 2' GMP | ||||||
 Components Components | RIBONUCLEASE T1 | ||||||
 Keywords Keywords |  ENDORIBONUCLEASE / ENDORIBONUCLEASE /  HYDROLASE / HYDROLASE /  NUCLEASE NUCLEASE | ||||||
| Function / homology |  Function and homology information Function and homology informationhyphal tip /  ribonuclease T1 activity / ribonuclease T1 activity /  ribonuclease T1 / cell septum / ribonuclease T1 / cell septum /  endonuclease activity / endonuclease activity /  lyase activity / lyase activity /  RNA binding RNA bindingSimilarity search - Function | ||||||
| Biological species |   Aspergillus oryzae (mold) Aspergillus oryzae (mold) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.8 Å X-RAY DIFFRACTION / Resolution: 1.8 Å | ||||||
 Authors Authors | Doumen, J. / Gonciarz, M. / Zegers, I. / Loris, R. / Wyns, L. / Steyaert, J. | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 1996 Journal: Protein Sci. / Year: 1996Title: A catalytic function for the structurally conserved residue Phe 100 of ribonuclease T1. Authors: Doumen, J. / Gonciarz, M. / Zegers, I. / Loris, R. / Wyns, L. / Steyaert, J. #1:  Journal: Biochemistry / Year: 1992 Journal: Biochemistry / Year: 1992Title: Role of Histidine-40 in Ribonuclease T1 Catalysis: Three-Dimensional Structures of the Partially Active His40Lys Mutant Authors: Zegers, I. / Verhelst, P. / Choe, H.W. / Steyaert, J. / Heinemann, U. / Saenger, W. / Wyns, L. #2:  Journal: J.Biol.Chem. / Year: 1988 Journal: J.Biol.Chem. / Year: 1988Title: Three-Dimensional Structure of the Ribonuclease T1 2'-Gmp Complex at 1.9-A Resolution Authors: Arni, R. / Heinemann, U. / Tokuoka, R. / Saenger, W. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1bir.cif.gz 1bir.cif.gz | 52 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1bir.ent.gz pdb1bir.ent.gz | 40.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1bir.json.gz 1bir.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bi/1bir https://data.pdbj.org/pub/pdb/validation_reports/bi/1bir ftp://data.pdbj.org/pub/pdb/validation_reports/bi/1bir ftp://data.pdbj.org/pub/pdb/validation_reports/bi/1bir | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given Matrix: (-0.9963, 0.0852, -0.0065), Vector  : : |
- Components
Components
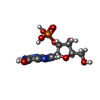
| #1: Protein |  / RNASE T1 / RNASE T1Mass: 11018.598 Da / Num. of mol.: 2 / Mutation: F100A Source method: isolated from a genetically manipulated source Details: COMPLEX WITH 2'-GMP / Source: (gene. exp.)   Aspergillus oryzae (mold) Aspergillus oryzae (mold)Description: STEYAERT ET AL. (1990) BIOCHEMISTRY 29, 9064-9072 Gene: SYNTHETIC GENE / Plasmid: PMC5-RT1 / Gene (production host): SYNTHETIC GENE / Production host:   Escherichia coli (E. coli) / References: UniProt: P00651, EC: 3.1.27.3 Escherichia coli (E. coli) / References: UniProt: P00651, EC: 3.1.27.3#2: Chemical | #3: Chemical |  Guanosine monophosphate Guanosine monophosphate#4: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.16 Å3/Da / Density % sol: 43.04 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | *PLUS pH: 4.2 / Method: vapor diffusion, sitting drop / Details: used to seeding | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction source | Wavelength: 1.5418 |
|---|---|
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1.5418 Å / Relative weight: 1 : 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→10 Å / Observed criterion σ(I): 0 / Redundancy: 3.8 % / Rmerge(I) obs: 0.056 |
| Reflection | *PLUS Highest resolution: 1.8 Å / Num. obs: 17890 / % possible obs: 99.7 % / Rmerge(I) obs: 0.047 |
| Reflection shell | *PLUS Highest resolution: 1.82 Å / Lowest resolution: 1.9 Å / % possible obs: 100 % / Rmerge(I) obs: 0.056 |
- Processing
Processing
| Software |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.8→10 Å / σ(F): 0
| ||||||||||||||||
| Displacement parameters | Biso mean: 14.84 Å2 | ||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→10 Å
| ||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Version: 3.1 / Classification: refinement X-PLOR / Version: 3.1 / Classification: refinement | ||||||||||||||||
| Refinement | *PLUS Rfactor Rfree : 0.24 : 0.24 | ||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller














 PDBj
PDBj