[English] 日本語
 Yorodumi
Yorodumi- EMDB-4742: Structural basis of Cullin-2 RING E3 ligase regulation by the COP... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4742 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural basis of Cullin-2 RING E3 ligase regulation by the COP9 signalosome | |||||||||
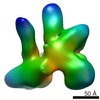
 Map data Map data | Structural basis of Cullin-2 RING E3 ligase regulation by the COP9 signalosome | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationnucleotide-excision repair factor 4 complex / regulation of DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator /  COP9 signalosome assembly / trophectodermal cell proliferation / COP9 signalosome assembly / trophectodermal cell proliferation /  macrophage migration inhibitory factor binding / global genome nucleotide-excision repair / regulation of IRE1-mediated unfolded protein response / exosomal secretion / deNEDDylase activity / GTPase inhibitor activity ...nucleotide-excision repair factor 4 complex / regulation of DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator / macrophage migration inhibitory factor binding / global genome nucleotide-excision repair / regulation of IRE1-mediated unfolded protein response / exosomal secretion / deNEDDylase activity / GTPase inhibitor activity ...nucleotide-excision repair factor 4 complex / regulation of DNA damage response, signal transduction by p53 class mediator resulting in transcription of p21 class mediator /  COP9 signalosome assembly / trophectodermal cell proliferation / COP9 signalosome assembly / trophectodermal cell proliferation /  macrophage migration inhibitory factor binding / global genome nucleotide-excision repair / regulation of IRE1-mediated unfolded protein response / exosomal secretion / deNEDDylase activity / GTPase inhibitor activity / eukaryotic translation initiation factor 3 complex / regulation of protein neddylation / protein deneddylation / cullin-RING-type E3 NEDD8 transferase / cellular response to chemical stress / NEDD8 transferase activity / cullin-RING ubiquitin ligase complex / macrophage migration inhibitory factor binding / global genome nucleotide-excision repair / regulation of IRE1-mediated unfolded protein response / exosomal secretion / deNEDDylase activity / GTPase inhibitor activity / eukaryotic translation initiation factor 3 complex / regulation of protein neddylation / protein deneddylation / cullin-RING-type E3 NEDD8 transferase / cellular response to chemical stress / NEDD8 transferase activity / cullin-RING ubiquitin ligase complex /  COP9 signalosome / Cul7-RING ubiquitin ligase complex / metal-dependent deubiquitinase activity / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / COP9 signalosome / Cul7-RING ubiquitin ligase complex / metal-dependent deubiquitinase activity / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway /  regulation of proteolysis / activation of NF-kappaB-inducing kinase activity / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling / target-directed miRNA degradation / elongin complex / VCB complex / positive regulation of protein autoubiquitination / protein neddylation / regulation of proteolysis / activation of NF-kappaB-inducing kinase activity / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling / target-directed miRNA degradation / elongin complex / VCB complex / positive regulation of protein autoubiquitination / protein neddylation /  Hydrolases; Acting on peptide bonds (peptidases) / NEDD8 ligase activity / Cul5-RING ubiquitin ligase complex / RHOBTB1 GTPase cycle / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / ubiquitin-ubiquitin ligase activity / Cul4A-RING E3 ubiquitin ligase complex / Cul2-RING ubiquitin ligase complex / Hydrolases; Acting on peptide bonds (peptidases) / NEDD8 ligase activity / Cul5-RING ubiquitin ligase complex / RHOBTB1 GTPase cycle / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / ubiquitin-ubiquitin ligase activity / Cul4A-RING E3 ubiquitin ligase complex / Cul2-RING ubiquitin ligase complex /  SCF ubiquitin ligase complex / inner cell mass cell proliferation / negative regulation of type I interferon production / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / Cul3-RING ubiquitin ligase complex / TP53 Regulates Transcription of DNA Repair Genes / Prolactin receptor signaling / protein deubiquitination / skeletal muscle cell differentiation / protein monoubiquitination / TGF-beta receptor signaling activates SMADs / cullin family protein binding / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / regulation of JNK cascade / response to light stimulus / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / anatomical structure morphogenesis / protein K48-linked ubiquitination / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / Formation of HIV elongation complex in the absence of HIV Tat / Nuclear events stimulated by ALK signaling in cancer / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / response to UV / JNK cascade / positive regulation of TORC1 signaling / RNA Polymerase II Pre-transcription Events / SCF ubiquitin ligase complex / inner cell mass cell proliferation / negative regulation of type I interferon production / Cul4B-RING E3 ubiquitin ligase complex / ubiquitin ligase complex scaffold activity / Cul3-RING ubiquitin ligase complex / TP53 Regulates Transcription of DNA Repair Genes / Prolactin receptor signaling / protein deubiquitination / skeletal muscle cell differentiation / protein monoubiquitination / TGF-beta receptor signaling activates SMADs / cullin family protein binding / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / regulation of JNK cascade / response to light stimulus / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / anatomical structure morphogenesis / protein K48-linked ubiquitination / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / Formation of HIV elongation complex in the absence of HIV Tat / Nuclear events stimulated by ALK signaling in cancer / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / response to UV / JNK cascade / positive regulation of TORC1 signaling / RNA Polymerase II Pre-transcription Events /  translation initiation factor activity / translation initiation factor activity /  Regulation of BACH1 activity / Regulation of BACH1 activity /  T cell activation / T cell activation /  post-translational protein modification / intrinsic apoptotic signaling pathway / transcription corepressor binding / Degradation of DVL / transcription elongation by RNA polymerase II / Recognition of DNA damage by PCNA-containing replication complex / post-translational protein modification / intrinsic apoptotic signaling pathway / transcription corepressor binding / Degradation of DVL / transcription elongation by RNA polymerase II / Recognition of DNA damage by PCNA-containing replication complex /  transcription initiation at RNA polymerase II promoter / nucleotide-excision repair / cellular response to amino acid stimulus / TP53 Regulates Transcription of DNA Repair Genes / Degradation of GLI1 by the proteasome / Negative regulation of NOTCH4 signaling / Iron uptake and transport / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Vif-mediated degradation of APOBEC3G / Hedgehog 'on' state / DNA Damage Recognition in GG-NER / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / G1/S transition of mitotic cell cycle / negative regulation of canonical Wnt signaling pathway / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / protein modification process / modification-dependent protein catabolic process / RING-type E3 ubiquitin transferase / Degradation of beta-catenin by the destruction complex / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / transcription initiation at RNA polymerase II promoter / nucleotide-excision repair / cellular response to amino acid stimulus / TP53 Regulates Transcription of DNA Repair Genes / Degradation of GLI1 by the proteasome / Negative regulation of NOTCH4 signaling / Iron uptake and transport / GSK3B and BTRC:CUL1-mediated-degradation of NFE2L2 / Vif-mediated degradation of APOBEC3G / Hedgehog 'on' state / DNA Damage Recognition in GG-NER / Degradation of GLI2 by the proteasome / GLI3 is processed to GLI3R by the proteasome / G1/S transition of mitotic cell cycle / negative regulation of canonical Wnt signaling pathway / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / protein modification process / modification-dependent protein catabolic process / RING-type E3 ubiquitin transferase / Degradation of beta-catenin by the destruction complex / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha /  protein localization / Inactivation of CSF3 (G-CSF) signaling protein localization / Inactivation of CSF3 (G-CSF) signalingSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 5.9 Å cryo EM / Resolution: 5.9 Å | |||||||||
 Authors Authors | Faull SF / Lau AMC / Beuron F / Cronin NB / Morris EP / Politis A | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Structural basis of Cullin 2 RING E3 ligase regulation by the COP9 signalosome. Authors: Sarah V Faull / Andy M C Lau / Chloe Martens / Zainab Ahdash / Kjetil Hansen / Hugo Yebenes / Carla Schmidt / Fabienne Beuron / Nora B Cronin / Edward P Morris / Argyris Politis /    Abstract: Cullin-Ring E3 Ligases (CRLs) regulate a multitude of cellular pathways through specific substrate receptors. The COP9 signalosome (CSN) deactivates CRLs by removing NEDD8 from activated Cullins. ...Cullin-Ring E3 Ligases (CRLs) regulate a multitude of cellular pathways through specific substrate receptors. The COP9 signalosome (CSN) deactivates CRLs by removing NEDD8 from activated Cullins. Here we present structures of the neddylated and deneddylated CSN-CRL2 complexes by combining single-particle cryo-electron microscopy (cryo-EM) with chemical cross-linking mass spectrometry (XL-MS). These structures suggest a conserved mechanism of CSN activation, consisting of conformational clamping of the CRL2 substrate by CSN2/CSN4, release of the catalytic CSN5/CSN6 heterodimer and finally activation of the CSN5 deneddylation machinery. Using hydrogen-deuterium exchange (HDX)-MS we show that CRL2 activates CSN5/CSN6 in a neddylation-independent manner. The presence of NEDD8 is required to activate the CSN5 active site. Overall, by synergising cryo-EM with MS, we identify sensory regions of the CSN that mediate its stepwise activation and provide a framework for understanding the regulatory mechanism of other Cullin family members. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4742.map.gz emd_4742.map.gz | 37.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4742-v30.xml emd-4742-v30.xml emd-4742.xml emd-4742.xml | 27.4 KB 27.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_4742.png emd_4742.png | 131.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4742 http://ftp.pdbj.org/pub/emdb/structures/EMD-4742 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4742 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4742 | HTTPS FTP |
-Related structure data
| Related structure data |  6r7iMC  4736C  4739C  4741C  4744C  6r6hC  6r7fC  6r7hC  6r7nC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4742.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4742.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structural basis of Cullin-2 RING E3 ligase regulation by the COP9 signalosome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : CSN-CRL2-N8
+Supramolecule #1: CSN-CRL2-N8
+Macromolecule #1: COP9 signalosome complex subunit 1
+Macromolecule #2: COP9 signalosome complex subunit 2
+Macromolecule #3: COP9 signalosome complex subunit 3
+Macromolecule #4: COP9 signalosome complex subunit 4
+Macromolecule #5: COP9 signalosome complex subunit 5
+Macromolecule #6: COP9 signalosome complex subunit 6
+Macromolecule #7: COP9 signalosome complex subunit 7b
+Macromolecule #8: COP9 signalosome complex subunit 8
+Macromolecule #9: NEDD8
+Macromolecule #10: Cullin-2
+Macromolecule #11: Elongin-B
+Macromolecule #12: Elongin-C
+Macromolecule #13: E3 ubiquitin-protein ligase RBX1
+Macromolecule #14: ZINC ION
+Macromolecule #15: water
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 5.0 nm / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 75.0 µm / Calibrated defocus max: 3.0 µm / Calibrated defocus min: 1.8 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm Bright-field microscopy / Cs: 2.7 mm |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 2 / Average electron dose: 40.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Software - Name: RELION |
|---|---|
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 5.9 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION / Number images used: 316921 |
 Movie
Movie Controller
Controller