+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | SaPI1 portal-capsid interface in mature capsids with DNA | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords |  capsid / capsid /  phage / sapi / portal / phage / sapi / portal /  STRUCTURAL PROTEIN STRUCTURAL PROTEIN | |||||||||||||||
| Biological species |  Dubowvirus dv80alpha Dubowvirus dv80alpha | |||||||||||||||
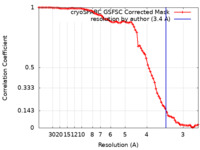
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.4 Å cryo EM / Resolution: 3.4 Å | |||||||||||||||
 Authors Authors | Kizziah JL / Mukherjee A / Dokland T | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2024 Journal: J Mol Biol / Year: 2024Title: Structure of the Portal Complex from Staphylococcus aureus Pathogenicity Island 1 Transducing Particles In Situ and In Isolation. Authors: Amarshi Mukherjee / James L Kizziah / N'Toia C Hawkins / Mohamed O Nasef / Laura K Parker / Terje Dokland /  Abstract: Staphylococcus aureus is an important human pathogen, and the prevalence of antibiotic resistance is a major public health concern. The evolution of pathogenicity and resistance in S. aureus often ...Staphylococcus aureus is an important human pathogen, and the prevalence of antibiotic resistance is a major public health concern. The evolution of pathogenicity and resistance in S. aureus often involves acquisition of mobile genetic elements (MGEs). Bacteriophages play an especially important role, since transduction represents the main mechanism for horizontal gene transfer. S. aureus pathogenicity islands (SaPIs), including SaPI1, are MGEs that carry genes encoding virulence factors, and are mobilized at high frequency through interactions with specific "helper" bacteriophages, such as 80α, leading to packaging of the SaPI genomes into virions made from structural proteins supplied by the helper. Among these structural proteins is the portal protein, which forms a ring-like portal at a fivefold vertex of the capsid, through which the DNA is packaged during virion assembly and ejected upon infection of the host. We have used high-resolution cryo-electron microscopy to determine structures of the S. aureus bacteriophage 80α portal itself, produced by overexpression, and in situ in the empty and full SaPI1 virions, and show how the portal interacts with the capsid. These structures provide a basis for understanding portal and capsid assembly and the conformational changes that occur upon DNA packaging and ejection. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43147.map.gz emd_43147.map.gz | 49.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43147-v30.xml emd-43147-v30.xml emd-43147.xml emd-43147.xml | 21 KB 21 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43147_fsc.xml emd_43147_fsc.xml | 7.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_43147.png emd_43147.png | 156.4 KB | ||
| Masks |  emd_43147_msk_1.map emd_43147_msk_1.map | 52.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43147.cif.gz emd-43147.cif.gz | 6 KB | ||
| Others |  emd_43147_additional_1.map.gz emd_43147_additional_1.map.gz emd_43147_additional_2.map.gz emd_43147_additional_2.map.gz emd_43147_half_map_1.map.gz emd_43147_half_map_1.map.gz emd_43147_half_map_2.map.gz emd_43147_half_map_2.map.gz | 45.9 MB 44.8 MB 48.2 MB 48.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43147 http://ftp.pdbj.org/pub/emdb/structures/EMD-43147 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43147 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43147 | HTTPS FTP |
-Related structure data
| Related structure data |  8vdeMC  8v8bC  8vd4C  8vd5C  8vd8C  8vdcC C: citing same article ( M: atomic model generated by this map |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43147.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43147.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.33 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43147_msk_1.map emd_43147_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: C12 symmetry averaged map resampled to main map...
| File | emd_43147_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | C12 symmetry averaged map resampled to main map used to constrain portal protein models in ISOLDE | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: C5 symmetry averaged map resampled to main map...
| File | emd_43147_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | C5 symmetry averaged map resampled to main map used to constrain capsid protein models in ISOLDE | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_43147_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_43147_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Dubowvirus dv80alpha
| Entire | Name:  Dubowvirus dv80alpha Dubowvirus dv80alpha |
|---|---|
| Components |
|
-Supramolecule #1: Dubowvirus dv80alpha
| Supramolecule | Name: Dubowvirus dv80alpha / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 53369 / Sci species name: Dubowvirus dv80alpha / Virus type: SATELLITE / Virus isolate: SPECIES / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: Major capsid protein
| Macromolecule | Name: Major capsid protein / type: protein_or_peptide / ID: 1 / Number of copies: 15 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Dubowvirus dv80alpha Dubowvirus dv80alpha |
| Molecular weight | Theoretical: 36.846883 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MEQTQKLKLN LQHFASNNVK PQVFNPDNVM MHEKKDGTLM NEFTTPILQE VMENSKIMQL GKYEPMEGTE KKFTFWADKP GAYWVGEGQ KIETSKATWV NATMRAFKLG VILPVTKEFL NYTYSQFFEE MKPMIAEAFY KKFDEAGILN QGNNPFGKSI A QSIEKTNK ...String: MEQTQKLKLN LQHFASNNVK PQVFNPDNVM MHEKKDGTLM NEFTTPILQE VMENSKIMQL GKYEPMEGTE KKFTFWADKP GAYWVGEGQ KIETSKATWV NATMRAFKLG VILPVTKEFL NYTYSQFFEE MKPMIAEAFY KKFDEAGILN QGNNPFGKSI A QSIEKTNK VIKGDFTQDN IIDLEALLED DELEANAFIS KTQNRSLLRK IVDPETKERI YDRNSDSLDG LPVVNLKSSN LK RGELITG DFDKLIYGIP QLIEYKIDET AQLSTVKNED GTPVNLFEQD MVALRATMHV ALHIADDKAF AKLVPADKRT DSV PGEV |
-Macromolecule #2: Portal protein
| Macromolecule | Name: Portal protein / type: protein_or_peptide / ID: 2 / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Dubowvirus dv80alpha Dubowvirus dv80alpha |
| Molecular weight | Theoretical: 59.55182 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MLKVNEFETD TDLRGNINYL FNDEANVVYT YDGTESDLLQ NVNEVSKYIE HHMDYQRPRL KVLSDYYEGK TKNLVELTRR KEEYMADNR VAHDYASYIS DFINGYFLGN PIQYQDDDKD VLEAIEAFND LNDVESHNRS LGLDLSIYGK AYELMIRNQD D ETRLYKSD ...String: MLKVNEFETD TDLRGNINYL FNDEANVVYT YDGTESDLLQ NVNEVSKYIE HHMDYQRPRL KVLSDYYEGK TKNLVELTRR KEEYMADNR VAHDYASYIS DFINGYFLGN PIQYQDDDKD VLEAIEAFND LNDVESHNRS LGLDLSIYGK AYELMIRNQD D ETRLYKSD AMSTFIIYDN TVERNSIAGV RYLRTKPIDK TDEDEVFTVD LFTSHGVYRY LTNRTNGLKL TPRENSFESH SF ERMPITE FSNNERRKGD YEKVITLIDL YDNAESDTAN YMSDLNDAML LIKGNLNLDP VEVRKQKEAN VLFLEPTVYV DAE GRETEG SVDGGYIYKQ YDVQGTEAYK DRLNSDIHMF TNTPNMKDDN FSGTQSGEAM KYKLFGLEQR TKTKEGLFTK GLRR RAKLL ETILKNTRSI DANKDFNTVR YVYNRNLPKS LIEELKAYID SGGKISQTTL MSLFSFFQDP ELEVKKIEED EKESI KKAQ KGIYKDPRDI NDDEQDDDTK DTVDKKE |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.7000000000000001 µm Bright-field microscopy / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.7000000000000001 µm |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 35.26 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z
Z Y
Y X
X