[English] 日本語
 Yorodumi
Yorodumi- EMDB-41816: Cryo-EM structure of the RAF1-HSP90-CDC37 complex in the closed state -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the RAF1-HSP90-CDC37 complex in the closed state | |||||||||
 Map data Map data | Sharpened Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | CRAF /  RAF1 / RAF1 /  HSP90 / HSP90 /  CDC37 / CDC37 /  SIGNALING PROTEIN-CHAPERONE complex SIGNALING PROTEIN-CHAPERONE complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of type II interferon-mediated signaling pathway / HSP90-CDC37 chaperone complex /  death-inducing signaling complex assembly / positive regulation of mitophagy in response to mitochondrial depolarization / intermediate filament cytoskeleton organization / type B pancreatic cell proliferation / protein kinase regulator activity / regulation of Rho protein signal transduction / SHOC2 M1731 mutant abolishes MRAS complex function / Gain-of-function MRAS complexes activate RAF signaling ...regulation of type II interferon-mediated signaling pathway / HSP90-CDC37 chaperone complex / death-inducing signaling complex assembly / positive regulation of mitophagy in response to mitochondrial depolarization / intermediate filament cytoskeleton organization / type B pancreatic cell proliferation / protein kinase regulator activity / regulation of Rho protein signal transduction / SHOC2 M1731 mutant abolishes MRAS complex function / Gain-of-function MRAS complexes activate RAF signaling ...regulation of type II interferon-mediated signaling pathway / HSP90-CDC37 chaperone complex /  death-inducing signaling complex assembly / positive regulation of mitophagy in response to mitochondrial depolarization / intermediate filament cytoskeleton organization / type B pancreatic cell proliferation / protein kinase regulator activity / regulation of Rho protein signal transduction / SHOC2 M1731 mutant abolishes MRAS complex function / Gain-of-function MRAS complexes activate RAF signaling / Rap1 signalling / death-inducing signaling complex assembly / positive regulation of mitophagy in response to mitochondrial depolarization / intermediate filament cytoskeleton organization / type B pancreatic cell proliferation / protein kinase regulator activity / regulation of Rho protein signal transduction / SHOC2 M1731 mutant abolishes MRAS complex function / Gain-of-function MRAS complexes activate RAF signaling / Rap1 signalling /  regulation of cell motility / protein folding chaperone complex / insulin secretion involved in cellular response to glucose stimulus / Negative feedback regulation of MAPK pathway / post-transcriptional regulation of gene expression / GP1b-IX-V activation signalling / IFNG signaling activates MAPKs / Drug-mediated inhibition of ERBB2 signaling / Resistance of ERBB2 KD mutants to trastuzumab / Resistance of ERBB2 KD mutants to sapitinib / Resistance of ERBB2 KD mutants to tesevatinib / Resistance of ERBB2 KD mutants to neratinib / Resistance of ERBB2 KD mutants to osimertinib / Resistance of ERBB2 KD mutants to afatinib / Resistance of ERBB2 KD mutants to AEE788 / Resistance of ERBB2 KD mutants to lapatinib / regulation of cell motility / protein folding chaperone complex / insulin secretion involved in cellular response to glucose stimulus / Negative feedback regulation of MAPK pathway / post-transcriptional regulation of gene expression / GP1b-IX-V activation signalling / IFNG signaling activates MAPKs / Drug-mediated inhibition of ERBB2 signaling / Resistance of ERBB2 KD mutants to trastuzumab / Resistance of ERBB2 KD mutants to sapitinib / Resistance of ERBB2 KD mutants to tesevatinib / Resistance of ERBB2 KD mutants to neratinib / Resistance of ERBB2 KD mutants to osimertinib / Resistance of ERBB2 KD mutants to afatinib / Resistance of ERBB2 KD mutants to AEE788 / Resistance of ERBB2 KD mutants to lapatinib /  Drug resistance in ERBB2 TMD/JMD mutants / ERBB2-ERBB3 signaling pathway / Drug resistance in ERBB2 TMD/JMD mutants / ERBB2-ERBB3 signaling pathway /  regulation of cell differentiation / face development / regulation of cyclin-dependent protein serine/threonine kinase activity / regulation of cell differentiation / face development / regulation of cyclin-dependent protein serine/threonine kinase activity /  pseudopodium / somatic stem cell population maintenance / neurotrophin TRK receptor signaling pathway / thyroid gland development / regulation of type I interferon-mediated signaling pathway / extrinsic apoptotic signaling pathway via death domain receptors / pseudopodium / somatic stem cell population maintenance / neurotrophin TRK receptor signaling pathway / thyroid gland development / regulation of type I interferon-mediated signaling pathway / extrinsic apoptotic signaling pathway via death domain receptors /  MAP kinase kinase kinase activity / MAP kinase kinase kinase activity /  protein targeting / negative regulation of protein-containing complex assembly / RHOBTB2 GTPase cycle / Schwann cell development / type II interferon-mediated signaling pathway / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / Signaling by ERBB2 / protein targeting / negative regulation of protein-containing complex assembly / RHOBTB2 GTPase cycle / Schwann cell development / type II interferon-mediated signaling pathway / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / Signaling by ERBB2 /  heat shock protein binding / response to muscle stretch / activation of adenylate cyclase activity / heat shock protein binding / response to muscle stretch / activation of adenylate cyclase activity /  myelination / CD209 (DC-SIGN) signaling / Constitutive Signaling by Overexpressed ERBB2 / insulin-like growth factor receptor signaling pathway / thymus development / ATP-dependent protein folding chaperone / Signaling by ERBB2 TMD/JMD mutants / myelination / CD209 (DC-SIGN) signaling / Constitutive Signaling by Overexpressed ERBB2 / insulin-like growth factor receptor signaling pathway / thymus development / ATP-dependent protein folding chaperone / Signaling by ERBB2 TMD/JMD mutants /  Hsp90 protein binding / RAF activation / Signaling by high-kinase activity BRAF mutants / Constitutive Signaling by EGFRvIII / Hsp90 protein binding / RAF activation / Signaling by high-kinase activity BRAF mutants / Constitutive Signaling by EGFRvIII /  wound healing / MAP2K and MAPK activation / negative regulation of cysteine-type endopeptidase activity involved in apoptotic process / Signaling by ERBB2 ECD mutants / Signaling by ERBB2 KD Mutants / Regulation of necroptotic cell death / Stimuli-sensing channels / Downregulation of ERBB2 signaling / wound healing / MAP2K and MAPK activation / negative regulation of cysteine-type endopeptidase activity involved in apoptotic process / Signaling by ERBB2 ECD mutants / Signaling by ERBB2 KD Mutants / Regulation of necroptotic cell death / Stimuli-sensing channels / Downregulation of ERBB2 signaling /  kinase binding / Negative regulation of MAPK pathway / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / kinase binding / Negative regulation of MAPK pathway / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants /  MAPK cascade / unfolded protein binding / Signaling by BRAF and RAF1 fusions / MAPK cascade / unfolded protein binding / Signaling by BRAF and RAF1 fusions /  protein folding / Constitutive Signaling by Ligand-Responsive EGFR Cancer Variants / insulin receptor signaling pathway / positive regulation of peptidyl-serine phosphorylation / protein-folding chaperone binding / protein folding / Constitutive Signaling by Ligand-Responsive EGFR Cancer Variants / insulin receptor signaling pathway / positive regulation of peptidyl-serine phosphorylation / protein-folding chaperone binding /  scaffold protein binding / regulation of apoptotic process / mitochondrial outer membrane / positive regulation of MAPK cascade / protein stabilization / scaffold protein binding / regulation of apoptotic process / mitochondrial outer membrane / positive regulation of MAPK cascade / protein stabilization /  non-specific serine/threonine protein kinase / non-specific serine/threonine protein kinase /  protein kinase activity / negative regulation of cell population proliferation / protein kinase activity / negative regulation of cell population proliferation /  protein phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / negative regulation of apoptotic process / protein phosphorylation / protein serine kinase activity / protein serine/threonine kinase activity / apoptotic process / negative regulation of apoptotic process /  protein kinase binding / protein kinase binding /  Golgi apparatus / Golgi apparatus /  enzyme binding / enzyme binding /  signal transduction / signal transduction /  ATP hydrolysis activity ATP hydrolysis activitySimilarity search - Function | |||||||||
| Biological species |   Trichoplusia ni (cabbage looper) / Trichoplusia ni (cabbage looper) /   Homo sapiens (human) Homo sapiens (human) | |||||||||
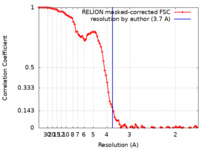
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.7 Å cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Finci LI / Simanshu DK | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2024 Journal: Commun Biol / Year: 2024Title: Structural dynamics of RAF1-HSP90-CDC37 and HSP90 complexes reveal asymmetric client interactions and key structural elements. Authors: Lorenzo I Finci / Mayukh Chakrabarti / Gulcin Gulten / Joseph Finney / Carissa Grose / Tara Fox / Renbin Yang / Dwight V Nissley / Frank McCormick / Dominic Esposito / Trent E Balius / Dhirendra K Simanshu /  Abstract: RAF kinases are integral to the RAS-MAPK signaling pathway, and proper RAF1 folding relies on its interaction with the chaperone HSP90 and the cochaperone CDC37. Understanding the intricate molecular ...RAF kinases are integral to the RAS-MAPK signaling pathway, and proper RAF1 folding relies on its interaction with the chaperone HSP90 and the cochaperone CDC37. Understanding the intricate molecular interactions governing RAF1 folding is crucial for comprehending this process. Here, we present a cryo-EM structure of the closed-state RAF1-HSP90-CDC37 complex, where the C-lobe of the RAF1 kinase domain binds to one side of the HSP90 dimer, and an unfolded N-lobe segment of the RAF1 kinase domain threads through the center of the HSP90 dimer. CDC37 binds to the kinase C-lobe, mimicking the N-lobe with its HxNI motif. We also describe structures of HSP90 dimers without RAF1 and CDC37, displaying only N-terminal and middle domains, which we term the semi-open state. Employing 1 μs atomistic simulations, energetic decomposition, and comparative structural analysis, we elucidate the dynamics and interactions within these complexes. Our quantitative analysis reveals that CDC37 bridges the HSP90-RAF1 interaction, RAF1 binds HSP90 asymmetrically, and that HSP90 structural elements engage RAF1's unfolded region. Additionally, N- and C-terminal interactions stabilize HSP90 dimers, and molecular interactions in HSP90 dimers rearrange between the closed and semi-open states. Our findings provide valuable insight into the contributions of HSP90 and CDC37 in mediating client folding. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41816.map.gz emd_41816.map.gz | 31.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41816-v30.xml emd-41816-v30.xml emd-41816.xml emd-41816.xml | 24.9 KB 24.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41816_fsc.xml emd_41816_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_41816.png emd_41816.png | 28.1 KB | ||
| Filedesc metadata |  emd-41816.cif.gz emd-41816.cif.gz | 7.6 KB | ||
| Others |  emd_41816_additional_1.map.gz emd_41816_additional_1.map.gz emd_41816_half_map_1.map.gz emd_41816_half_map_1.map.gz emd_41816_half_map_2.map.gz emd_41816_half_map_2.map.gz | 49.4 MB 49.7 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41816 http://ftp.pdbj.org/pub/emdb/structures/EMD-41816 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41816 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41816 | HTTPS FTP |
-Related structure data
| Related structure data |  8u1lMC  8u1mC  8u1nC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41816.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41816.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened Map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.858 Å | ||||||||||||||||||||
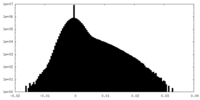
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Unsharpened Map
| File | emd_41816_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
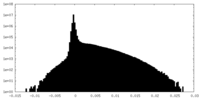
| Density Histograms |
-Half map: Unfiltered, unsharpened, Halfmap 1
| File | emd_41816_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered, unsharpened, Halfmap 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
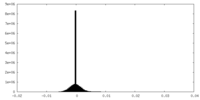
| Density Histograms |
-Half map: Unfiltered, unsharpened, Halfmap 2
| File | emd_41816_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered, unsharpened, Halfmap 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
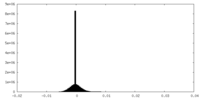
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of RAF1-HSP90-CDC37
| Entire | Name: Complex of RAF1-HSP90-CDC37 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of RAF1-HSP90-CDC37
| Supramolecule | Name: Complex of RAF1-HSP90-CDC37 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: Heat shock protein 90
| Supramolecule | Name: Heat shock protein 90 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
-Supramolecule #3: RAF1 & HSP90 co-chaperone CDC37
| Supramolecule | Name: RAF1 & HSP90 co-chaperone CDC37 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Heat shock protein 83
| Macromolecule | Name: Heat shock protein 83 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Molecular weight | Theoretical: 83.322133 KDa |
| Sequence | String: MPEEMQTDSG EVETFAFQAE IAQLMSLIIN TFYSNKEIFL RELISNSSDA LDKIRYESLT DPSKLDSGKE LYIKIIPNKS EGTFTIIDT GIGMTKADLV NNLGTIAKSG TKAFMEALQA GADISMIGQF GVGFYSCYLV ADRVTVHSKH NDDEQYMWES S AGGSFTVR ...String: MPEEMQTDSG EVETFAFQAE IAQLMSLIIN TFYSNKEIFL RELISNSSDA LDKIRYESLT DPSKLDSGKE LYIKIIPNKS EGTFTIIDT GIGMTKADLV NNLGTIAKSG TKAFMEALQA GADISMIGQF GVGFYSCYLV ADRVTVHSKH NDDEQYMWES S AGGSFTVR TDHGEPLGRG TKIVLHIKED LAEYLEVNKI KEIVKKHSQF IGYPIKLTVE KEREKELAYD EEEEKKEGEE DK KEDEKED EKPKIEDVGE DDEEDKDKKK KKTIKEKYTE DEELNKTKPI WTRNADDITQ EEYGDFYKSL TNDWEDHLAV KHF SVEGQL EFRALLFVPR RAPFDLFENK KRKNNIKLYV RRVFIMDNCE DLIPEYLNFI KGVVDSEDLP LNISREMLQQ NKIL KVIRK NLVKKCLELF EELAEDKENY KKYYEQFSKN LKLGIHEDAQ NRTKLADLLR YHTSASGDEA CSLKEYVSRM KENQK HIYY ITGENRDQVA NSSFVERVKK RGYEVVYMTE PIDEYVVQQM REYDGKTLVS VTKEGLELPE DEEEKKKREE DKVKFE GLC KVMKNILDNK VEKVVVSNRL VESPCCIVTA QYGWSANMER IMKAQALRDT STMGYMAAKK HLEINPDHSI VETLRQK AE ADKNDKAVKD LVILLYETAL LSSGFTLDEP QVHASRIYRM IKLGLGIDED EPIQVEESSV GDVPPLEGDA DDASRMEE V D UniProtKB:  Heat shock protein 83 Heat shock protein 83 |
-Macromolecule #2: RAF proto-oncogene serine/threonine-protein kinase
| Macromolecule | Name: RAF proto-oncogene serine/threonine-protein kinase / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 74.021445 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: GGEHIQGAWK TISNGFGFKD AVFDGSSCIS PTIVQQFGYQ RRASDDGKLT DPSKTSNTIR VFLPNKQRTV VNVRNGMSLH DCLMKALKV RGLQPECCAV FRLLHEHKGK KARLDWNTDA ASLIGEELQV DFLDHVPLTT HNFARKTFLK LAFCDICQKF L LNGFRCQT ...String: GGEHIQGAWK TISNGFGFKD AVFDGSSCIS PTIVQQFGYQ RRASDDGKLT DPSKTSNTIR VFLPNKQRTV VNVRNGMSLH DCLMKALKV RGLQPECCAV FRLLHEHKGK KARLDWNTDA ASLIGEELQV DFLDHVPLTT HNFARKTFLK LAFCDICQKF L LNGFRCQT CGYKFHEHCS TKVPTMCVDW SNIRQLLLFP NSTIGDSGVP ALPSLTMRRM RESVSRMPVS SQHRYSTPHA FT FNTSSPS SEGSLSQRQR STSTPNVHMV STTLPVDSRM IEDAIRSHSE SASPSALSSS PNNLSPTGWS QPKTPVPAQR ERA PVSGTQ EKNKIRPRGQ RDSSYYWEIE ASEVMLSTRI GSGSFGTVYK GKWHGDVAVK ILKVVDPTPE QFQAFRNEVA VLRK TRHVN ILLFMGYMTK DNLAIVTQWC EGSSLYKHLH VQETKFQMFQ LIDIARQTAQ GMDYLHAKNI IHRDMKSNNI FLHEG LTVK IGDFGLATVK SRWSGSQQVE QPTGSVLWMA PEVIRMQDNN PFSFQSDVYS YGIVLYELMT GELPYSHINN RDQIIF MVG RGYASPDLSK LYKNCPKAMK RLVADCVKKV KEERPLFPQI LSSIELLQHS LPKINRSASE PSLHRAAHTE DINACTL TT SPRLPVFGHH HHHH UniProtKB: RAF proto-oncogene serine/threonine-protein kinase |
-Macromolecule #3: Hsp90 co-chaperone Cdc37, N-terminally processed
| Macromolecule | Name: Hsp90 co-chaperone Cdc37, N-terminally processed / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 44.622363 KDa |
| Recombinant expression | Organism:   Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MVDYSVWDHI EV(SEP)DDEDETH PNIDTASLFR WRHQARVERM EQFQKEKEEL DRGCRECKRK VAECQRKLKE LEVAEG GKA ELERLQAEAQ QLRKEERSWE QKLEEMRKKE KSMPWNVDTL SKDGFSKSMV NTKPEKTEED SEEVREQKHK TFVEKYE KQ IKHFGMLRRW ...String: MVDYSVWDHI EV(SEP)DDEDETH PNIDTASLFR WRHQARVERM EQFQKEKEEL DRGCRECKRK VAECQRKLKE LEVAEG GKA ELERLQAEAQ QLRKEERSWE QKLEEMRKKE KSMPWNVDTL SKDGFSKSMV NTKPEKTEED SEEVREQKHK TFVEKYE KQ IKHFGMLRRW DDSQKYLSDN VHLVCEETAN YLVIWCIDLE VEEKCALMEQ VAHQTIVMQF ILELAKSLKV DPRACFRQ F FTKIKTADRQ YMEGFNDELE AFKERVRGRA KLRIEKAMKE YEEEERKKRL GPGGLDPVEV YESLPEELQK CFDVKDVQM LQDAISKMDP TDAKYHMQRC IDSGLWVPNS KASEAKEGEE AGPGDPLLEA VPKTGDEKDV SV UniProtKB: Hsp90 co-chaperone Cdc37 |
-Macromolecule #4: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 2 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.3 Component:
| |||||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR | |||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV Details: Grids were blotted for 4.5 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm Bright-field microscopy / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z
Z Y
Y X
X