[English] 日本語
 Yorodumi
Yorodumi- EMDB-40856: Single particle reconstruction of the human LINE-1 ORF2p without ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
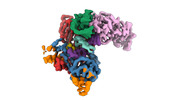
| Title | Single particle reconstruction of the human LINE-1 ORF2p without substrate (apo) | |||||||||
 Map data Map data | Main map of apo ORF2p core | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  reverse transcriptase / reverse transcriptase /  LINE-1 / LINE-1 /  RNA BINDING PROTEIN RNA BINDING PROTEIN | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 4.06 Å cryo EM / Resolution: 4.06 Å | |||||||||
 Authors Authors | van Eeuwen T / Taylor MS / Rout MP | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Structures, functions and adaptations of the human LINE-1 ORF2 protein. Authors: Eric T Baldwin / Trevor van Eeuwen / David Hoyos / Arthur Zalevsky / Egor P Tchesnokov / Roberto Sánchez / Bryant D Miller / Luciano H Di Stefano / Francesc Xavier Ruiz / Matthew Hancock / ...Authors: Eric T Baldwin / Trevor van Eeuwen / David Hoyos / Arthur Zalevsky / Egor P Tchesnokov / Roberto Sánchez / Bryant D Miller / Luciano H Di Stefano / Francesc Xavier Ruiz / Matthew Hancock / Esin Işik / Carlos Mendez-Dorantes / Thomas Walpole / Charles Nichols / Paul Wan / Kirsi Riento / Rowan Halls-Kass / Martin Augustin / Alfred Lammens / Anja Jestel / Paula Upla / Kera Xibinaku / Samantha Congreve / Maximiliaan Hennink / Kacper B Rogala / Anna M Schneider / Jennifer E Fairman / Shawn M Christensen / Brian Desrosiers / Gregory S Bisacchi / Oliver L Saunders / Nafeeza Hafeez / Wenyan Miao / Rosana Kapeller / Dennis M Zaller / Andrej Sali / Oliver Weichenrieder / Kathleen H Burns / Matthias Götte / Michael P Rout / Eddy Arnold / Benjamin D Greenbaum / Donna L Romero / John LaCava / Martin S Taylor /      Abstract: The LINE-1 (L1) retrotransposon is an ancient genetic parasite that has written around one-third of the human genome through a 'copy and paste' mechanism catalysed by its multifunctional enzyme, open ...The LINE-1 (L1) retrotransposon is an ancient genetic parasite that has written around one-third of the human genome through a 'copy and paste' mechanism catalysed by its multifunctional enzyme, open reading frame 2 protein (ORF2p). ORF2p reverse transcriptase (RT) and endonuclease activities have been implicated in the pathophysiology of cancer, autoimmunity and ageing, making ORF2p a potential therapeutic target. However, a lack of structural and mechanistic knowledge has hampered efforts to rationally exploit it. We report structures of the human ORF2p 'core' (residues 238-1061, including the RT domain) by X-ray crystallography and cryo-electron microscopy in several conformational states. Our analyses identified two previously undescribed folded domains, extensive contacts to RNA templates and associated adaptations that contribute to unique aspects of the L1 replication cycle. Computed integrative structural models of full-length ORF2p show a dynamic closed-ring conformation that appears to open during retrotransposition. We characterize ORF2p RT inhibition and reveal its underlying structural basis. Imaging and biochemistry show that non-canonical cytosolic ORF2p RT activity can produce RNA:DNA hybrids, activating innate immune signalling through cGAS/STING and resulting in interferon production. In contrast to retroviral RTs, L1 RT is efficiently primed by short RNAs and hairpins, which probably explains cytosolic priming. Other biochemical activities including processivity, DNA-directed polymerization, non-templated base addition and template switching together allow us to propose a revised L1 insertion model. Finally, our evolutionary analysis demonstrates structural conservation between ORF2p and other RNA- and DNA-dependent polymerases. We therefore provide key mechanistic insights into L1 polymerization and insertion, shed light on the evolutionary history of L1 and enable rational drug development targeting L1. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40856.map.gz emd_40856.map.gz | 61.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40856-v30.xml emd-40856-v30.xml emd-40856.xml emd-40856.xml | 22.9 KB 22.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40856_fsc.xml emd_40856_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_40856.png emd_40856.png | 36 KB | ||
| Masks |  emd_40856_msk_1.map emd_40856_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-40856.cif.gz emd-40856.cif.gz | 6.8 KB | ||
| Others |  emd_40856_additional_1.map.gz emd_40856_additional_1.map.gz emd_40856_half_map_1.map.gz emd_40856_half_map_1.map.gz emd_40856_half_map_2.map.gz emd_40856_half_map_2.map.gz | 117 MB 116.2 MB 116.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40856 http://ftp.pdbj.org/pub/emdb/structures/EMD-40856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40856 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40856.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40856.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map of apo ORF2p core | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.65 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_40856_msk_1.map emd_40856_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: 4.5 angstrom lowpass filtered map
| File | emd_40856_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 4.5 angstrom lowpass filtered map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_40856_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_40856_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Long Interspersed Nuclear Element (LINE)-1 ORF2 protein
| Entire | Name: Long Interspersed Nuclear Element (LINE)-1 ORF2 protein |
|---|---|
| Components |
|
-Supramolecule #1: Long Interspersed Nuclear Element (LINE)-1 ORF2 protein
| Supramolecule | Name: Long Interspersed Nuclear Element (LINE)-1 ORF2 protein type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: LINE-1 retrotransposable element ORF2 protein
| Macromolecule | Name: LINE-1 retrotransposable element ORF2 protein / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MTGSNSHITI LTLNVNGLNS PIKRHRLASW IKSQDPSVCC IQETHLTCRD THRLKIKGWR KIYQANGKQK KAGVAILVSD KTDFKPTKIK RDKEGHYIMV KGSIQQEELT ILNIYAPNTG APRFIKQVLS DLQRDLDSHT LIMGDFNTPL SILDRSTRQK VNKDTQELNS ...String: MTGSNSHITI LTLNVNGLNS PIKRHRLASW IKSQDPSVCC IQETHLTCRD THRLKIKGWR KIYQANGKQK KAGVAILVSD KTDFKPTKIK RDKEGHYIMV KGSIQQEELT ILNIYAPNTG APRFIKQVLS DLQRDLDSHT LIMGDFNTPL SILDRSTRQK VNKDTQELNS ALHQTDLIDI YRTLHPKSTE YTFFSAPHHT YSKIDHIVGS KALLSKCKRT EIITNYLSDH SAIKLELRIK NLTQSRSTTW KLNNLLLNDY WVHNEMKAEI KMFFETNENK DTTYQNLWDA FKAVCRGKFI ALNAYKRKQE RSKIDTLTSQ LKELEKQEQT HSKASRRQEI TKIRAELKEI ETQKTLQKIN ESRSWFFERI NKIDRPLARL IKKKREKNQI DTIKNDKGDI TTDPTEIQTT IREYYKHLYA NKLENLEEMD TFLDTYTLPR LNQEEVESLN RPITGSEIVA IINSLPTKKS PGPDGFTAEF YQRYKEELVP FLLKLFQSIE KEGILPNSFY EASIILIPKP GRDTTKKENF RPISLMNIDA KILNKILANR IQQHIKKLIH HDQVGFIPGM QGWFNIRKSI NVIQHINRAK DKNHVIISID AEKAFDKIQQ PFMLKTLNKL GIDGMYLKII RAIYDKPTAN IILNGQKLEA FPLKTGTRQG CPLSPLLFNI VLEVLARAIR QEKEIKGIQL GKEEVKLSLF ADDMIVYLEN PIVSAQNLLK LISNFSKVSG YKINVQKSQA FLYNNNRQTE SQIMGELPFT IASKRIKYLG IQLTRDVKDL FKENYKPLLK EIKEDTNKWK NIPCSWVGRI NIVKMAILPK VIYRFNAIPI KLPMTFFTEL EKTTLKFIWN QKRARIAKSI LSQKNKAGGI TLPDFKLYYK ATVTKTAWYW YQNRDIDQWN RTEPSEIMPH IYNYLIFDKP EKNKQWGKDS LLNKWCWENW LAICRKLKLD PFLTPYTKIN SRWIKDLNVK PKTIKTLEEN LGITIQDIGV GKDFMSKTPK AMATKDKIDK WDLIKLKSFC TAKETTIRVN RQPTTWEKIF ATYSSDKGLI SRIYNELKQI YKKKTNNPIK KWAKDMNRHF SKEDIYAAKK HMKKCSSSLA IREMQIKTTM RYHLTPVRMA IIKKSGNNRC WRGCGEIGTL VHCWWDCKLV QPLWKSVWRF LRDLELEIPF DPAIPLLGIY PKDYKSCCYK DTCTRMFIAA LFTIAKTWNQ PNCPTMIDWI KKMWHIYTME YYAAIKNDEF ISFVGTWMKL ETIILSKLSQ EQKTKHRIFS LIGGN |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.6 Component:
Details: 20mM HEPES pH 7.6, 150mM NaCl, 2mM MgOAc, 2mM DTT, 2mM dTTP | |||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Instrument: LEICA EM CPC / Details: Manually blotted from the back. | |||||||||||||||
| Details | Sample was monodisperse though unstable |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 130000 Bright-field microscopy / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 6411 / Average electron dose: 51.0 e/Å2 Details: Super-resolution images collected in dose-fractionation mode over 38 frames wiht a dose per frame of 1.32 e/A2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X


































