Yorodumi
Yorodumi+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of AMPA receptor GluA2 complex with auxiliary subunits TARP gamma-5 and cornichon-2 bound to competitive antagonist ZK, channel blocker spermidine and antiepileptic drug perampanel (closed state) | |||||||||||||||
 Map data Map data | Structure of LBD-TMD of AMPA receptor GluA2 in complex with auxiliary subunits TARP gamma-5 and cornichon-2 bound to competitive antagonist ZK, channel blocker spermidine and antiepileptic drug PMP | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords |  AMPA receptor / AMPA receptor /  spermidine / spermidine /  perampanel / perampanel /  cornichon-2 / cornichon-2 /  SIGNALING PROTEIN SIGNALING PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationneurotransmitter receptor transport, postsynaptic endosome to lysosome /  regulation of AMPA receptor activity / neurotransmitter receptor internalization / channel regulator activity / Cargo concentration in the ER / postsynaptic neurotransmitter receptor diffusion trapping / COPII-mediated vesicle transport / spine synapse / dendritic spine neck / dendritic spine head ...neurotransmitter receptor transport, postsynaptic endosome to lysosome / regulation of AMPA receptor activity / neurotransmitter receptor internalization / channel regulator activity / Cargo concentration in the ER / postsynaptic neurotransmitter receptor diffusion trapping / COPII-mediated vesicle transport / spine synapse / dendritic spine neck / dendritic spine head ...neurotransmitter receptor transport, postsynaptic endosome to lysosome /  regulation of AMPA receptor activity / neurotransmitter receptor internalization / channel regulator activity / Cargo concentration in the ER / postsynaptic neurotransmitter receptor diffusion trapping / COPII-mediated vesicle transport / spine synapse / dendritic spine neck / dendritic spine head / Activation of AMPA receptors / response to lithium ion / perisynaptic space / cellular response to glycine / transmission of nerve impulse / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors / regulation of AMPA receptor activity / neurotransmitter receptor internalization / channel regulator activity / Cargo concentration in the ER / postsynaptic neurotransmitter receptor diffusion trapping / COPII-mediated vesicle transport / spine synapse / dendritic spine neck / dendritic spine head / Activation of AMPA receptors / response to lithium ion / perisynaptic space / cellular response to glycine / transmission of nerve impulse / AMPA glutamate receptor activity / Trafficking of GluR2-containing AMPA receptors /  immunoglobulin binding / AMPA glutamate receptor complex / kainate selective glutamate receptor activity / immunoglobulin binding / AMPA glutamate receptor complex / kainate selective glutamate receptor activity /  ionotropic glutamate receptor complex / extracellularly glutamate-gated ion channel activity / asymmetric synapse / regulation of receptor recycling / Unblocking of NMDA receptors, glutamate binding and activation / ionotropic glutamate receptor complex / extracellularly glutamate-gated ion channel activity / asymmetric synapse / regulation of receptor recycling / Unblocking of NMDA receptors, glutamate binding and activation /  voltage-gated calcium channel activity / voltage-gated calcium channel activity /  glutamate receptor binding / positive regulation of synaptic transmission / glutamate-gated receptor activity / presynaptic active zone membrane / response to fungicide / cellular response to brain-derived neurotrophic factor stimulus / glutamate receptor binding / positive regulation of synaptic transmission / glutamate-gated receptor activity / presynaptic active zone membrane / response to fungicide / cellular response to brain-derived neurotrophic factor stimulus /  regulation of synaptic transmission, glutamatergic / vesicle-mediated transport / somatodendritic compartment / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / regulation of synaptic transmission, glutamatergic / vesicle-mediated transport / somatodendritic compartment / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential /  ionotropic glutamate receptor binding / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm / endoplasmic reticulum-Golgi intermediate compartment membrane / ionotropic glutamate receptor binding / ionotropic glutamate receptor signaling pathway / dendrite cytoplasm / endoplasmic reticulum-Golgi intermediate compartment membrane /  cytoskeletal protein binding / positive regulation of synaptic transmission, glutamatergic / cytoskeletal protein binding / positive regulation of synaptic transmission, glutamatergic /  SNARE binding / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / dendritic shaft / SNARE binding / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / dendritic shaft /  synaptic membrane / synaptic membrane /  synaptic transmission, glutamatergic / synaptic transmission, glutamatergic /  PDZ domain binding / postsynaptic density membrane / protein tetramerization / modulation of chemical synaptic transmission / Schaffer collateral - CA1 synapse / establishment of protein localization / ER to Golgi transport vesicle membrane / PDZ domain binding / postsynaptic density membrane / protein tetramerization / modulation of chemical synaptic transmission / Schaffer collateral - CA1 synapse / establishment of protein localization / ER to Golgi transport vesicle membrane /  terminal bouton / terminal bouton /  receptor internalization / synaptic vesicle membrane / cerebral cortex development / receptor internalization / synaptic vesicle membrane / cerebral cortex development /  synaptic vesicle / presynapse / synaptic vesicle / presynapse /  presynaptic membrane / presynaptic membrane /  signaling receptor activity / signaling receptor activity /  amyloid-beta binding / amyloid-beta binding /  growth cone / growth cone /  perikaryon / chemical synaptic transmission / perikaryon / chemical synaptic transmission /  scaffold protein binding / scaffold protein binding /  postsynaptic membrane / postsynaptic membrane /  dendritic spine / dendritic spine /  postsynaptic density / neuron projection / postsynaptic density / neuron projection /  axon / neuronal cell body / axon / neuronal cell body /  dendrite / dendrite /  synapse / glutamatergic synapse / protein-containing complex binding / endoplasmic reticulum membrane / synapse / glutamatergic synapse / protein-containing complex binding / endoplasmic reticulum membrane /  protein kinase binding / protein kinase binding /  cell surface / cell surface /  endoplasmic reticulum / protein-containing complex / endoplasmic reticulum / protein-containing complex /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | |||||||||||||||
| Biological species |   Rattus norvegicus (Norway rat) / Rattus norvegicus (Norway rat) /   Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
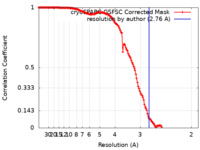
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 2.76 Å cryo EM / Resolution: 2.76 Å | |||||||||||||||
 Authors Authors | Gangwar SP / Yen LY / Yelshanskaya MV / Sobolevsky AI | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Modulation of GluA2-γ5 synaptic complex desensitization, polyamine block and antiepileptic perampanel inhibition by auxiliary subunit cornichon-2. Authors: Shanti Pal Gangwar / Laura Y Yen / Maria V Yelshanskaya / Aryeh Korman / Drew R Jones / Alexander I Sobolevsky /  Abstract: Synaptic complexes of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) with auxiliary subunits mediate most excitatory neurotransmission and can be targeted to treat ...Synaptic complexes of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) with auxiliary subunits mediate most excitatory neurotransmission and can be targeted to treat neuropsychiatric and neurological disorders, including epilepsy. Here we present cryogenic-electron microscopy structures of rat GluA2 AMPAR complexes with inhibitory mouse γ5 and potentiating human cornichon-2 (CNIH2) auxiliary subunits. CNIH2 appears to destabilize the desensitized state of the complex by reducing the separation of the upper lobes in ligand-binding domain dimers. At the same time, CNIH2 stabilizes binding of polyamine spermidine to the selectivity filter of the closed ion channel. Nevertheless, CNIH2, and to a lesser extent γ5, attenuate polyamine block of the open channel and reduce the potency of the antiepileptic drug perampanel that inhibits the synaptic complex allosterically by binding to sites in the ion channel extracellular collar. These findings illustrate the fine-tuning of synaptic complex structure and function in an auxiliary subunit-dependent manner, which is critical for the study of brain region-specific neurotransmission and design of therapeutics for disease treatment. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40746.map.gz emd_40746.map.gz | 259.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40746-v30.xml emd-40746-v30.xml emd-40746.xml emd-40746.xml | 22.1 KB 22.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40746_fsc.xml emd_40746_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_40746.png emd_40746.png | 71.7 KB | ||
| Filedesc metadata |  emd-40746.cif.gz emd-40746.cif.gz | 7.5 KB | ||
| Others |  emd_40746_half_map_1.map.gz emd_40746_half_map_1.map.gz emd_40746_half_map_2.map.gz emd_40746_half_map_2.map.gz | 255 MB 255 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40746 http://ftp.pdbj.org/pub/emdb/structures/EMD-40746 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40746 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40746 | HTTPS FTP |
-Related structure data
| Related structure data |  8ss7MC  8bhfC  8ss2C  8ss3C  8ss4C  8ss5C  8ss6C  8ss8C  8ss9C  8ssaC  8ssbC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40746.map.gz / Format: CCP4 / Size: 274.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40746.map.gz / Format: CCP4 / Size: 274.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of LBD-TMD of AMPA receptor GluA2 in complex with auxiliary subunits TARP gamma-5 and cornichon-2 bound to competitive antagonist ZK, channel blocker spermidine and antiepileptic drug PMP | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.925 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_40746_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_40746_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : GluA2-TARP gamma5-cornichon-2
| Entire | Name: GluA2-TARP gamma5-cornichon-2 |
|---|---|
| Components |
|
-Supramolecule #1: GluA2-TARP gamma5-cornichon-2
| Supramolecule | Name: GluA2-TARP gamma5-cornichon-2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 Details: Structure of LBD-TMD of AMPA receptor GluA2 in complex with auxiliary subunits TARP gamma-5 and cornichon-2 bound to competitive antagonist ZK, channel blocker spermidine and antiepileptic ...Details: Structure of LBD-TMD of AMPA receptor GluA2 in complex with auxiliary subunits TARP gamma-5 and cornichon-2 bound to competitive antagonist ZK, channel blocker spermidine and antiepileptic drug perampanel (closed state) |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
-Macromolecule #1: Glutamate receptor 2, Voltage-dependent calcium channel gamma-5 s...
| Macromolecule | Name: Glutamate receptor 2, Voltage-dependent calcium channel gamma-5 subunit chimera type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
| Molecular weight | Theoretical: 114.978859 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NSIQIGGLFP RGADQEYSAF RVGMVQFSTS EFRLTPHIDN LEVANSFAVT NAFCSQFSRG VYAIFGFYDK KSVNTITSFC GTLHVSFIT PSFPTDGTHP FVIQMRPDLK GALLSLIEYY QWDKFAYLYD SDRGLSTLQA VLDSAAEKKW QVTAINVGNI N NDKKDETY ...String: NSIQIGGLFP RGADQEYSAF RVGMVQFSTS EFRLTPHIDN LEVANSFAVT NAFCSQFSRG VYAIFGFYDK KSVNTITSFC GTLHVSFIT PSFPTDGTHP FVIQMRPDLK GALLSLIEYY QWDKFAYLYD SDRGLSTLQA VLDSAAEKKW QVTAINVGNI N NDKKDETY RSLFQDLELK KERRVILDCE RDKVNDIVDQ VITIGKHVKG YHYIIANLGF TDGDLLKIQF GGAEVSGFQI VD YDDSLVS KFIERWSTLE EKEYPGAHTA TIKYTSALTY DAVQVMTEAF RNLRKQRIEI SRRGNAGDCL ANPAVPWGQG VEI ERALKQ VQVEGLSGNI KFDQNGKRIN YTINIMELKT NGPRKIGYWS EVDKMVLTED DTSGLEQKTV VVTTILESPY VMMK KNHEM LEGNERYEGY CVDLAAEIAK HCGFKYKLTI VGDGKYGARD ADTKIWNGMV GELVYGKADI AIAPLTITLV REEVI DFSK PFMSLGISIM IKKPQKSKPG VFSFLDPLAY EIWMCIVFAY IGVSVVLFLV SRFSPYEWHT EEFEDGRETQ SSESTN EFG IFNSLWFSLG AFMQQGCDIS PRSLSGRIVG GVWWFFTLII ISSYTANLAA FLTVERMVSP IESAEDLSKQ TEIAYGT LD SGSTKEFFRR SKIAVFDKMW TYMRSAEPSV FVRTTAEGVA RVRKSKGKYA YLLESTMNEY IEQRKPCDTM KVGGNLDS K GYGIATPKGS SLGTPVNLAV LKLSEQGVLD KLKNKWWYDK GECGAKDSGS KEKTSALSLS NVAGVFYILV GGLGLAMLV ALIEFCYKSR AEAKRMKGTG SACGRKALTL LSSVFAVCGL GLLGIAVSTD YWLYLEEGII LPQNQSTEVK MSLHSGLWRV CFLAGEERG RCFTIEYVMP MNSQMTSEST VNVLKMIRSA TPFPLVSLFF MFIGFILSNI GHIRPHRTIL AFVSGIFFIL S GLSLVVGL VLYISSINDE MLNRTKDAET YFNYKYGWSF AFAAISFLLT ESAGVMSVYL FMKRYTAE UniProtKB:  Glutamate receptor 2, Voltage-dependent calcium channel gamma-5 subunit Glutamate receptor 2, Voltage-dependent calcium channel gamma-5 subunit |
-Macromolecule #2: Protein cornichon homolog 2
| Macromolecule | Name: Protein cornichon homolog 2 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.94842 KDa |
| Recombinant expression | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAFTFAAFCY MLTLVLCASL IFFVIWHIIA FDELRTDFKN PIDQGNPARA RERLKNIERI CCLLRKLVVP EYSIHGLFCL MFLCAAEWV TLGLNIPLLF YHLWRYFHRP ADGSEVMYDA VSIMNADILN YCQKESWCKL AFYLLSFFYY LYSMVYTLVS F UniProtKB: Protein cornichon homolog 2 |
-Macromolecule #3: 2-(6'-oxo-1'-phenyl[1',6'-dihydro[2,3'-bipyridine]]-5'-yl)benzonitrile
| Macromolecule | Name: 2-(6'-oxo-1'-phenyl[1',6'-dihydro[2,3'-bipyridine]]-5'-yl)benzonitrile type: ligand / ID: 3 / Number of copies: 4 / Formula: 6ZP |
|---|---|
| Molecular weight | Theoretical: 349.385 Da |
| Chemical component information | 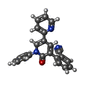 ChemComp-6ZP: |
-Macromolecule #4: {[7-morpholin-4-yl-2,3-dioxo-6-(trifluoromethyl)-3,4-dihydroquino...
| Macromolecule | Name: {[7-morpholin-4-yl-2,3-dioxo-6-(trifluoromethyl)-3,4-dihydroquinoxalin-1(2H)-yl]methyl}phosphonic acid type: ligand / ID: 4 / Number of copies: 4 / Formula: ZK1 |
|---|---|
| Molecular weight | Theoretical: 409.254 Da |
| Chemical component information | 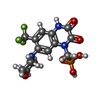 ChemComp-ZK1: |
-Macromolecule #5: 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1,2-DIOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 5 / Number of copies: 28 / Formula: PCW |
|---|---|
| Molecular weight | Theoretical: 787.121 Da |
| Chemical component information |  ChemComp-PCW: |
-Macromolecule #6: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 6 / Number of copies: 8 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #7: SODIUM ION
| Macromolecule | Name: SODIUM ION / type: ligand / ID: 7 / Number of copies: 1 |
|---|---|
| Molecular weight | Theoretical: 22.99 Da |
-Macromolecule #8: SPERMIDINE
| Macromolecule | Name: SPERMIDINE / type: ligand / ID: 8 / Number of copies: 1 / Formula: SPD |
|---|---|
| Molecular weight | Theoretical: 145.246 Da |
| Chemical component information |  ChemComp-SPD: |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.5 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: UltrAuFoil / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 25 sec. / Pretreatment - Atmosphere: AIR | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: vitrification carried out in nitrogen atmosphere. | |||||||||||||||
| Details | Protein extracted and purified in detergent micelle |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Software | Name:  Coot Coot |
|---|---|
| Output model |  PDB-8ss7: |
 Movie
Movie Controller
Controller



















 Z
Z Y
Y X
X