+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Monomeric MapSPARTA bound with guide RNA and target DNA hybrid | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | gRNA mediated DNA binding / Microbiology /  Argonaute / microbic immune system / Argonaute / microbic immune system /  IMMUNE SYSTEM IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) | |||||||||
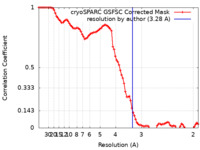
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.28 Å cryo EM / Resolution: 3.28 Å | |||||||||
 Authors Authors | Shen ZF / Yang XY / Fu TM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Oligomerization-mediated activation of a short prokaryotic Argonaute. Authors: Zhangfei Shen / Xiao-Yuan Yang / Shiyu Xia / Wei Huang / Derek J Taylor / Kotaro Nakanishi / Tian-Min Fu /  Abstract: Although eukaryotic and long prokaryotic Argonaute proteins (pAgos) cleave nucleic acids, some short pAgos lack nuclease activity and hydrolyse NAD(P) to induce bacterial cell death. Here we present ...Although eukaryotic and long prokaryotic Argonaute proteins (pAgos) cleave nucleic acids, some short pAgos lack nuclease activity and hydrolyse NAD(P) to induce bacterial cell death. Here we present a hierarchical activation pathway for SPARTA, a short pAgo consisting of an Argonaute (Ago) protein and TIR-APAZ, an associated protein. SPARTA progresses through distinct oligomeric forms, including a monomeric apo state, a monomeric RNA-DNA-bound state, two dimeric RNA-DNA-bound states and a tetrameric RNA-DNA-bound active state. These snapshots together identify oligomerization as a mechanistic principle of SPARTA activation. The RNA-DNA-binding channel of apo inactive SPARTA is occupied by an auto-inhibitory motif in TIR-APAZ. After the binding of RNA-DNA, SPARTA transitions from a monomer to a symmetric dimer and then an asymmetric dimer, in which two TIR domains interact through charge and shape complementarity. Next, two dimers assemble into a tetramer with a central TIR cluster responsible for hydrolysing NAD(P). In addition, we observe unique features of interactions between SPARTA and RNA-DNA, including competition between the DNA 3' end and the auto-inhibitory motif, interactions between the RNA G2 nucleotide and Ago, and splaying of the RNA-DNA duplex by two loops exclusive to short pAgos. Together, our findings provide a mechanistic basis for the activation of short pAgos, a large section of the Ago superfamily. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40713.map.gz emd_40713.map.gz | 31.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40713-v30.xml emd-40713-v30.xml emd-40713.xml emd-40713.xml | 18.7 KB 18.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40713_fsc.xml emd_40713_fsc.xml | 8.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_40713.png emd_40713.png | 65.3 KB | ||
| Others |  emd_40713_additional_1.map.gz emd_40713_additional_1.map.gz emd_40713_half_map_1.map.gz emd_40713_half_map_1.map.gz emd_40713_half_map_2.map.gz emd_40713_half_map_2.map.gz | 56.5 MB 59.4 MB 59.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40713 http://ftp.pdbj.org/pub/emdb/structures/EMD-40713 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40713 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40713 | HTTPS FTP |
-Related structure data
| Related structure data |  8squMC  8fexC  8ffiC  8sp0C  8sp3C  8spoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_40713.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40713.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.95 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: sharped map
| File | emd_40713_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharped map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_40713_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_40713_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of MapSPARTA binding with gRNA tDNA hybrid
| Entire | Name: Complex of MapSPARTA binding with gRNA tDNA hybrid |
|---|---|
| Components |
|
-Supramolecule #1: Complex of MapSPARTA binding with gRNA tDNA hybrid
| Supramolecule | Name: Complex of MapSPARTA binding with gRNA tDNA hybrid / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) |
-Macromolecule #1: TIR-APAZ
| Macromolecule | Name: TIR-APAZ / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) |
| Molecular weight | Theoretical: 53.139398 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: RNKIFISHAT PDDNDFTRWL ALKLIGLGYE VWCDILFLDK GVDFWSNIEK VIREDTCKFL LVSSSYSNQR EGVLKELAVA AKVKKQLKD DKFIIPLAID EQLSYDDINI DIVRLNAIDF KMSWARGLKD ILEAFEKQKV PKEVADASKS NLLYQQIFLH D KSVIEKEE ...String: RNKIFISHAT PDDNDFTRWL ALKLIGLGYE VWCDILFLDK GVDFWSNIEK VIREDTCKFL LVSSSYSNQR EGVLKELAVA AKVKKQLKD DKFIIPLAID EQLSYDDINI DIVRLNAIDF KMSWARGLKD ILEAFEKQKV PKEVADASKS NLLYQQIFLH D KSVIEKEE IYDSNWLSIL SFPEELRFHE YNWMLPKRFD VRELTFPAVR YKNYLCTFAW AYDFTYHLPK TETYHKSKTI RI PTEEILS GSYDSNFIRN AECKRLIVQL LNKAFELRMK DKEVQEYEMS NKTAYWLEKG KLEKDKFEKT MLVGKQKDKN WHF AISGAS KLYPFPVLMI SSHIFFTADG KKLIDSSSVQ HSSRRRQGKN WWNNTWRTKL LAFIKYLSDD DTSFYLEMGS EEKV FVSNE PVKFKGNVSY NIPEKNTLEE EAELSGFNQG EDIEELEELI ENLEAE UniProtKB: TIR domain-containing protein |
-Macromolecule #2: short pAgo
| Macromolecule | Name: short pAgo / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) |
| Molecular weight | Theoretical: 58.09141 KDa |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: MKELIYIEEP KILFAHGQKC TDARDGLALF GPLNNLYGIK SGVIGTKQGL KIFRDYLDHI QKPIYNSNSI TRPMFPGFEA VFDCKWEST GITFKEVTNE DIGKFLYNSS THKRTYDLVS LFIDKIISAN KNEDENVDVW FVIVPDEIYK YCRPNSVLPK E MVQTKALM ...String: MKELIYIEEP KILFAHGQKC TDARDGLALF GPLNNLYGIK SGVIGTKQGL KIFRDYLDHI QKPIYNSNSI TRPMFPGFEA VFDCKWEST GITFKEVTNE DIGKFLYNSS THKRTYDLVS LFIDKIISAN KNEDENVDVW FVIVPDEIYK YCRPNSVLPK E MVQTKALM SKSKAKSFRY EPSLFPDINI ELKEQEKEAE TYNYDAQFHD QFKARLLKHT IPTQIFREST LAWRDFKNAF GL PIRDFSK IEGHLAWTIS TAAFYKAGGK PWKLSDVRNG VCYLGLVYKK VEKSKNPRNA CCAAQMFLDN GDGTVFKGEV GPW YNPKNG QYHLEPKEAK ALLSQSLQSY KEQIGEYPKE VFIHAKTRFN HQEWDAFLEV TPKETNLVGV TISKTKPLKL YKTE GDYTI LRGNAYVVNE RSAFLWTVGY VPKIQTALSM EVPNPLFIEI NKGEADIKQV LKDILSLTKL NYNACIFADG EPVTL RFAD KIGEILTAST DIKTPPLAFK YYI UniProtKB: Piwi domain-containing protein |
-Macromolecule #3: guide RNA
| Macromolecule | Name: guide RNA / type: rna / ID: 3 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) |
| Molecular weight | Theoretical: 6.651949 KDa |
| Sequence | String: UGACGGCUCU AAUCUAUUAG U |
-Macromolecule #4: target DNA
| Macromolecule | Name: target DNA / type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Maribacter polysiphoniae (bacteria) Maribacter polysiphoniae (bacteria) |
| Molecular weight | Theoretical: 7.675 KDa |
| Sequence | String: (DC)(DA)(DA)(DC)(DT)(DA)(DA)(DT)(DA)(DG) (DA)(DT)(DT)(DA)(DG)(DA)(DG)(DC)(DC)(DG) (DT)(DC)(DA)(DA)(DT) |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.5 µm Bright-field microscopy / Nominal defocus max: 2.1 µm / Nominal defocus min: 0.5 µm |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z
Z Y
Y X
X